Sarcoid Heart Disease: A Case Report and Literature Review
Fayez EL Shaer1,2*, Shahd Al-Awad1, Abdulmohsen Alghamdi1, Amer AlFakih1, Hamdan Al Shehri1.3, Mostafa Alshimiri1
1 King Fahad Cardiac Center, Department of Cardiac Sciences, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
2 National Heart Institute, Cairo, Egypt.
3Assistant Professor of Internal Medicine, Najran University, College of Medicine, Internal Medicine Department, Saudi Arabia.
ABSTRACT
Sarcoid Heart Disease (SHD), often misdiagnosed as tuberculosis is a potentially life-threatening condition. Cardiac manifestation of sarcoidosis is a challenging clinical condition as it is associated with pericarditis, heart block, ventricular arrhythmias, valve dysfunction, ventricular aneurysm, sudden cardiac death, and heart failure. This study presents a case of a syncopal attack due to complete heart block, which is the first indication of SHD. The study has also presented a literature review on the various management options employed for therapeutic and effective management of SHD. The study has proposed treatment approaches, such as Cardiac Magnetic Resonance (CMR), Magnetic Resonance Imaging (MRI), and Insertion of an Implantable Cardioverter Defibrillators (ICD) in patients with complete heart block to improve the quality of life and increase overall survival rates.
Key words: Arrhythmias, Cardiac Sarcoidosis, Cardiomyopathy, Heart Block
INTRODUCTION
Sarcoidosis is a multisystem inflammatory disorder of unknown etiology characterized by the presence of tiny lumps of cells in organs known as granulomas. There are numerous sites involved in sarcoidosis including lungs, skin, eyes, parotid gland, spleen, liver, and heart. Granulomas are an aggregation of macrophages having an epithelioid like appearance surrounded by a rim of lymphocytes observed in response to various inflammatory triggers throughout the body organs [1] Presentation of granulomas cannot be considered as the only symptom to diagnose Sarcoidosis, as many diseases such as Tuberculosis and Berylliosis also cause granulomatous inflammation [2-4]. Thus, Sarcoidosis requires correct clinical diagnosis and proper radiological and clinicopathological correlation in treatment [5].
Cardiac Sarcoidosis (CS) is found worldwide but most cases are reported in northern Europeans and African Americans, particularly in women [6]. However, the prevalence of sarcoidosis is potentially underestimated since many individuals with this disease have nonspecific symptoms. This disease affects patients from different regions and ages, with a prevalence of about 4.7 to 64 in 100,000 [6, 7]. The occurrence of sarcoidosis varies from the absence of symptoms demonstrating abnormal imaging to the heart failure and syncope due to arrhythmias resulting in sudden cardiac arrest [7]. It can also be presented in the forms of systemic complaints, pulmonary complaints, Löfgren syndrome (i.e., fever, bilateral hilar lymphadenopathy, and polyarthralgia) or dermatologic, ocular, and cardiac manifestations [5, 8].
Arrhythmia, also known as dysrhythmia results in either too fast, too slow heartbeat, or irregular heart rhythm [9]. Arrhythmia has two types: tachycardia (fast heartbeat) and bradycardia (slow heartbeat) [10]. SHD can result in both types of arrhythmias due to granuloma formation in the myocardium. This condition may result in a complete heart block (23-30%), bundle branch block (12-32%), ventricular tachycardia (23%), congestive heart failure (25-75%), and sudden death (25-65%) [11]. Sarcoid infiltration involves the cardiac structure, mainly myocardium in the left ventricular free wall and papillary muscle, basal septal wall, free wall of the right ventricle, and aria [12].
The diagnosis of cardiac sarcoidosis has improved with the development of cardiac magnetic resonance. In cardiac sarcoidosis, both atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are released indicating the presence of the disease. Approximately 25% of patients have non-caseation granulomas during autopsy with less than 5% having a clinical cardiac manifestation [13]. Ventricular tachyarrhythmia is a life-threatening condition with an incident of 15% per year. It can be managed by a corticosteroid in the early stage; while, intra-cardiac defibrillator (ICD) is required in advanced stage [14, 15].
More than 75% of the patients respond to symptomatic treatment with non-steroidal anti-inflammatory drugs (NSAIDs); while, patients with persistent pulmonary tuberculosis (15%) or extra-pulmonary tuberculosis (10%) require corticosteroid therapy [16]. These medicines can be administered by either one mg/kg per day or 40 mg per day dosage with tapering over several weeks [17]. Methotrexate (MTX) can be used as a corticosteroid-sparing strategy in chronic sarcoidosis for patients who are resistant to steroids or have side effects from steroids [18, 19]. Patients with SHD are monitored every 6-12 months to check their pulmonary function and chest radiography by an annual slit-lamp eye examination and ECGs [20]. This study has presented a case to examine the findings and management of a patient with sarcoid heart disease admitted to the cardiac center with a complete heart block.
Case Study
A 45-year-old man, working as a car maintenance technician, was presented at the emergency department with a history of syncopal attack, which occurred on the morning of the same day. The patient suffers from type II diabetes mellitus, hypertension, and is on regular medication; however, he was experiencing dizziness since the last 2 days. For the last 4 days, the patient had also experienced exertional chest pain, mainly in the left parasternal region characterized by heaviness with no radiation. The patient had also experienced shortness of breath (as per the New York Heart Association (NYHA), classification II), since the last 2 months.
Physical examination of the patient revealed the normal heartbeat with a regular heart rate of 43 b/m, no edema in lower limbs, intact peripheral pulsation; while, jugular venous pressure showed occasional Cannon wave.
Table 1. Lab Reports
|
Chest |
Vascular breathing |
|
Abdomen |
Unremarkable |
|
Vitals |
Vitals: T: 36.5 °C (Oral) |
|
Heart rate |
43 (Monitored) |
|
RR |
21 |
|
Blood Pressure |
181 / 83 |
|
SpO2 |
98% |
|
Weight |
118 kg |
|
WBC |
8,500 |
|
Hemoglobin |
14.5 |
|
Platelet |
190.0 |
|
Na |
137 |
|
Creatinine |
86 |
|
Urea |
4.5 |
|
Magnesium |
0.85 |
|
Phosphorus |
1.49 |
|
Ca |
2.47 |
|
INR |
1.11 |
|
Total CK |
60 |
|
Lactic acid |
2.7 |
|
ALT |
35.0 |
|
AST |
15 |
|
Troponin-I |
<0.01 |
|
APTT |
39.20 |
|
PPD |
< 5 mm |
|
Quantiferon test |
Indeterminate |
Echocardiography (ECHO) showed a 3rd-degree atrioventricular (AV) nodal block at a rate of 43 b/m with a narrow QRS complex (Figure 1a). The patient was admitted as a case syncope due to complete heart block with uncontrolled hypertension. In the emergency room, the patient was managed by atropine and a transcutaneous pacemaker together, with intensifying oral antihypertensive medication. CXR showed bilateral hilar lymphadenopathy as well as small bilateral pulmonary infiltration that predominantly involved mid-upper lung zones (Figure 1b). This further strengthened sarcoidosis as a strong differential diagnosis.
Pulmonary function test (PFT) demonstrated a restrictive lung defect in which there was a decrease in FEV1 and FVC, however, the FEV1/FVC ratio was >80%, which further supported the preliminary hypothesis of interstitial restrictive lung disease. ECHO showed normal LV and RV cavity size, wall thickness, normal overall systolic function (estimated LVEF 55-60%), and impaired relaxation with no regional wall motion abnormalities (Figure 2). The echocardiography showed no significant valvulopathy or underlying restrictive cardiomyopathy.
Cardiac catheterization was conducted to rule out coronary artery disease revealing almost normal coronary angiography with no flow-limiting lesion (Figure 3). The exercise test was conducted to detect the response of the high-grade heart block, which revealed worsening of heart block with a widening of QRS duration and no increase in heart rate. Cardiac MRI showed basal septal gadolinium enhancement (Figure 4).
CT report of the chest showed multiple indeterminate lung nodules (Figure 5a). High-resolution CT thorax (HRCT) showed established patterns of fibrosis in a honeycombing pattern with reticular opacities. CT abdomen showed evidence of hepatosplenomegaly with multiple Granulomatous lesions involving the liver and spleen (Figure 5b). This is considered as the main diagnosis of sarcoidosis; however, other possibilities such as lymphoma or fungal infection may also be possible. Endobronchial ultrasound, transbronchial needle aspiration biopsy (EBUS-TBNA) was performed on the hilar nodes showing the evidence of non-caseating granulomatous inflammation. In addition, biopsy samples were sent to microbiology for culture and sensitivity to rule out any other possible infectious etiologies of granulomatous inflammation.
During admission, the patient developed two episodes of electrical pauses. The first one lasted 5.5 seconds while he was asleep; whereas, the second pause was for 7.8 seconds. The patient was completely asymptomatic despite these episodes. Cardiac sarcoidosis was diagnosed on a clinical basis with basal septal gadolinium enhancement, together with the presence of extra-cardiac manifestation, and exclusion of tuberculosis. The patient was started on Prednisone 60mg PO OD with GI prophylaxis, insulin for diabetes control, and angiotensin blocker combination with calcium channel blocker. The patient clinically improved but still was in a complete heart block.
Thereafter, he developed a ventricular tachycardia while planned for permanent pacemaker insertion. Dual-Chamber ICD with pacing capability was implanted. Meanwhile, the patient had another attack of non-sustained ventricular tachycardia. Beta-blocker initiated together with the reprogramming of the ICD, while he continued on corticosteroid 60mg by the rheumatologist. The patient was then discharged and followed in the outpatient clinic by the rheumatologist for gradual tapering of steroids and electrophysiology clinic.
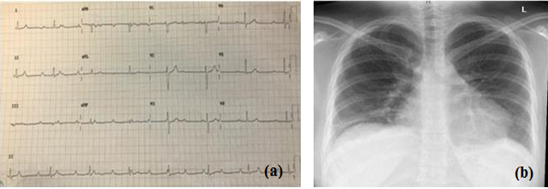
Figure 1: (a) ECG of the patient; (b) X-ray of the patient’s chest
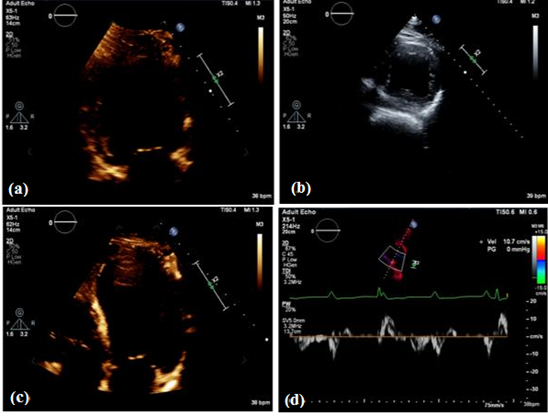
Figure 2: An ECHO view; (a) An ECHO 2 chamber view; (b) An ECHO short axis view; (c); (d) An ECHO 4-chamber view
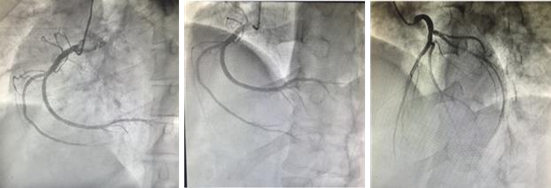
Figure 3: The cardiac catheterization of the normal coronaries
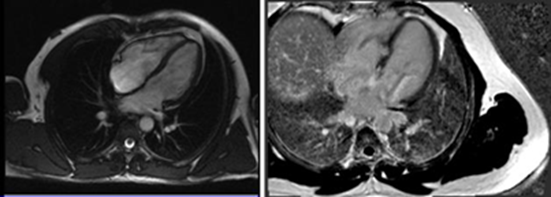
Figure 4: CMR gadolinium enhancement of the septum
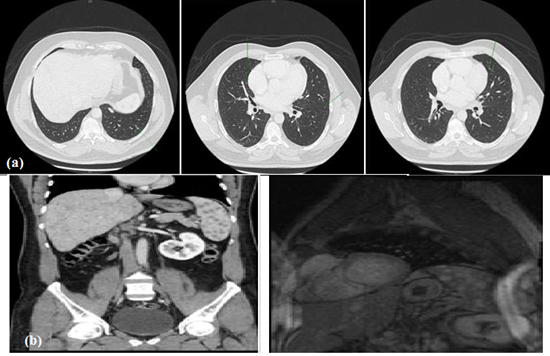
Figure 5: (a) CT chest scan; (b) CT abdomen scan
DISCUSSION
Cardiac involvement in sarcoidosis is a challenging clinical condition with an unpredictable course and progressive chronicity. Approximately, 20-30% of sarcoid patients have heart lesions; while, less than 5% of subjects are presented with clinical manifestations [19, 21]. Awareness about such cases is necessary due to the rarity of cardiac manifestation for rapid diagnosis and management. This requires a special strategy and specific algorithm to manage cost expenditure and shorten the length of hospital stay.
In the current case, the first presentation of sarcoid heart disease was a complete heart block in a young patient. A number of different diagnoses were warranted before establishing the actual diagnosis to rule out ischemic heart disease, valvular heart disease, myocardial disease, and cardiomyopathy. Such cases with complete heart block initially require ECG examination followed by CMR examination, arrhythmia assessment using Holter monitoring and stress testing, and assessment of extra-cardiac and systemic manifestations [22].
Focal myocardial sarcoid granuloma results in disturbed automaticity, which further causes the reoccurrence of tachyarrhythmia. The ventricular tachycardia (VT) is considered as the most common recurring kind of arrhythmia [23]. Roberts et al. [24] found that arrhythmia causes sudden death as a terminal event in sarcoid heart disease in 67% cases. However, supra-ventricular arrhythmias occur in 15-17% of cases resulting in atrial dilatation and/or pulmonary involvement due to atrial granuloma. About 23-30% of patients were reported with complete heart block after granuloma of the basal septum with scar formation or ischemia, which involves the atrioventricular nodal artery [11, 13, 25].
Around 25% of cases of cardiac sarcoidosis are followed by progressive congestive heart failure. This occurs due to extensive myocardial involvement with the formation of a ventricular aneurysm, pulmonary hypertension due to pulmonary involvement (core Pulmonale), and valve regurgitation or a combination of all [26]. Other clinical presentations of sarcoid heart disease vary from chest pain, impersonation of an acute coronary syndrome, acute pericarditis with pericardial effusion, and cardiac tamponade to constrictive pericarditis [27].
Laboratory tests have demonstrated many biomarkers of sarcoidosis such as Serum Amyloid A (SAA), Angiotensin-Converting Enzyme (ACE), the Glycoprotein KL-6 and soluble interleukin-2 receptor (sIL-2R), Chitotriosidase (CTO), Lysozyme, etc. [28]. In addition, hypercalcemia is a common laboratory finding in sarcoidosis resulting from non-caseating granuloma secreting excess 1,25-dihydroxy vitamin D3 [29]. Cardio-pulmonary exercise studies are requested to assess the peak oxygen consumption in case of heart failure. This assists doctors to examine reasons for dyspnea and fatigue to precisely differentiate between cardiac and pulmonary disorders. It also helps to optimize the decision-making process and outcome prediction, while determining targets for therapies [30]. ECHO is inefficient in determining which patient is more likely to develop high-risk arrhythmias, however, abnormalities can be detected in the advanced stage in 14% cases [11].
These abnormalities can range from diastolic dysfunction, systolic dysfunction with regional wall motion abnormalities, ventricular aneurysms, valve regurgitation, and pericardial effusion. Cardiac lesions can imitate hypertrophic cardiomyopathy as it may cause localized hypertrophy of the septal wall. However, it is missed at the early disease stage due to the lack of sensitivity of 2D echocardiography. Tissue characterization using backscatter that detects the acoustic character of the myocardium has a sensitivity of 75% in the early detection of SHD [31].
A gallium‐67 scintigraphy test is positive when the disease is active as it will accumulate only in an area of active inflammation. It is useful to detect the presence or absence of inflammation and response to corticosteroid therapy [11]. Positron emission tomography (PET) 18F‐FDG is more sensitive for myocardial involvement compared to thallium 201 SPECT and gallium-67 scintigraphy. The specification is not established as the heterogeneous uptake of FDG may occur in cases with idiopathic dilated cardiomyopathy [11].
CMR is highly sensitive in detecting early myocardial involvement, reliable in terms of assessing cardiac anatomy, function, inflammation, stress perfusion-fibrosis, aortic distensibility, and iron and fat deposition. Therefore, it constitutes an excellent tool for early diagnosis of cardiovascular involvement, risk stratification, treatment evaluation, and long-term follow-up of patients. Smedema et al. [32] revealed that sensitivity and specificity of CMR were 100% and 78%, respectively in detecting sarcoid heart disease.
Cardiac catheterization is indicated in patients with sarcoidosis in case of chest pain or heart failure to rule out associated coronary artery disease [13]. Transvenous endomyocardial biopsy is highly specific with low sensitivity due to patchy myocardial involvement resulting from sample error [11]. At autopsy, it was found that 25% of patients had non-caseating granulomas; while, less than 55% had a clinical cardiac manifestation [13].
In 1999, the National Institutes of Health’s a Case-Control Etiology of Sarcoidosis Study published the first-ever set of criteria for the diagnosis of CS [33]. It was followed by publishing a revised guideline by the Japanese Ministry of Health and Welfare (JMHW) criteria in 2007 [34]. Later in 2014, the Heart Rhythm Society in collaboration with multiple other societies published their first international guideline for the diagnosis of CS [35].
According to JMHW, cardiac sarcoidosis can be established when a histopathologic endomyocardial biopsy revealed non-caseating epithelioid granuloma. However, if the biopsy fails to show that the diagnosis can be supported by extracardiac sarcoidosis either histologically or clinically to satisfy the other criteria. Major criteria such as advanced heart block, basal thinning of the interventricular septum, positive myocardial gallium uptake, or ejection fraction less than 50%. Whereas, minor criteria like electrocardiographic criteria as arrhythmias, presence of wall motion abnormalities, or reduced myocardial thallium uptake or the presence of basal septal late gadolinium enhancement, and if the myocardial biopsy showed interstitial fibrosis or monocyte infiltration more than a moderate degree [34].
Most clinicians prefer corticosteroids as the first choice for treatment for symptomatic pulmonary sarcoidosis. Prednisone is one of the most commonly used corticosteroids, with the usual initial dose being 20–40 mg daily [36]. Yazaki [31] proved that a starting dose of 30 mg/day is enough for a better prognosis. After 2-3 months, the patients should be re-evaluated to assess the response of the disease to the therapy. Afterward, gradual tapering of the dose to be continued at a dose of 10-15 mg daily for 6 months. The tapering should be discontinued if the disease is under control after the subsequent evaluation [11]. Subsequent evaluation is done after 1-3 months and if the disease is under control, the dosage is reduced to 5-15 mg daily to be continued for 9-12 months [37]. Studies also confirm that no difference between higher doses than 50 mg compared to doses of less than 30 mg daily. The patient should be followed for at least 3 years to rule out relapse and ensure complete recovery [38]. Corticosteroid therapy halts the deterioration of LV function and maintains the normal LV systolic function. It can also improve mild to moderate LV systolic dysfunction [38]. However, an observational study showed no improvement in severe LV systolic dysfunction; whereas, there was improvement in ejection fraction (<35%), with no improvement in milder LV systolic dysfunction [39].
Non-corticosteroid agents such as methotrexate, azathioprine, or cyclophosphamide can be used in refractory cases necessitating a high dose of corticosteroid complicated with an adverse effect [39]. Antiarrhythmic beta-blockers are used to treat tachyarrhythmia; however, there is no clear evidence-based medicine in patients with sarcoidosis. Amiodarone is used as a broad-spectrum anti-arrhythmic, however, it can cause lung fibrosis [11]. An intra-cardiac defibrillator is recommended in the case of non-sustained ventricular tachycardia as there is a high chance for recurrence despite anti-arrhythmic therapy and corticosteroid [24]. Similarly, an ICD with pacing capability was inserted for our patient for the high-grade AV block. Cardiac transplantation is very rare but still an option in end-stage heart failure with recurrent persistent ventricular tachycardia especially in younger patients [11].
Data related to mortality is relatively limited. In the United States, death tends to mainly result from end-stage lung disease and right-side heart failure [40]. Independent predictors of mortality are functional status class (NYHA), left ventricular end-diastolic diameter, and arrhythmias (VT). Poor prognosis occurs with the worsening of left ventricular systolic function and functional status [31]. Corticosteroid therapy is vital as it improves outcomes, decreases ventricular arrhythmias, and even lead to a complete recovery of atrioventricular conduction [41].
CONCLUSION
This study has presented a literature review and a case report providing a detailed account of SHD and its management. The study found that the diagnosis of cardiac sarcoidosis follows a procedure beginning from echocardiography; while, ending at either cardiac transplantation, ICD, or death. The asymptomatic condition or mixed symptoms are accounted for the limitation of the successful diagnosis of SHD.
With the high index of suspicion, it is highly recommended to follow a specific algorithm for diagnosis and management of cardiac sarcoidosis, which will be more cost-effective and shorten hospital stay. Both, cardiac and systemic magnetic resonance imaging should be done as early as possible when sarcoidosis was suspected. Corticosteroid should be started as early as possible upon establishing the diagnosis of sarcoidosis. Intra-cardiac defibrillator with pacing capability considered early when arrhythmia detected due to a high chance for sudden cardiac death. Follow up is required of these patients every 6 months by chest X-ray, pulmonary function test, electrocardiography, as well as annual Slit-lamp eye examination.
ACKNOWLEDGMENT
The author is very thankful to all the associated personnel in any reference that contributed in/for the purpose of this research. This research is not funded by any resource.
REFERENCES
- Ahmadzai H, Huang S, Steinfort C, Markos J, Allen R, Wakefield D, Wilsher M, Thomas PS. State of the art paper on Sarcoidosis: An Educational Resource. The Thoracic Society of Australia & New Zealand. April 2018. Doi: https://doi.org/10.5694/mja17.00610
- Rahmanian V, Rahmanian K, Mansoorian E, Rahmanian N, Shakeri H, Rastgoofard MA. Prevalence of active tuberculosis & MTB-infection among diabetic population in southern of Iran, 2016. Pharmacophores. 2018;9(3):30-6.
- Korzh IV, Romanko TA, Zhirova IV, Podgaina MV, Tereschenko LV, Kalaycheva SG. Study of social and Epidemiological Indicators of tuberculosis in the European region. J. Adv. Pharm. Educ. Res. 2019;9(3):62-7.
- Bahmanjeh A, Kachooei SA, Ghasemi MF, Mosavari N, Hassanzadeh SM. Study on differentiation of pathogen-nonpathogen Mycobacterial infections using ESAT6-CFP10 in ELISA system. Arch. Pharm. Pract. 2020;11(2):28-36.
- Ungprasert P, Ryu JH, Matteson EL. Clinical Manifestations, Diagnosis, and Treatment of Sarcoidosis. Mayo Clinic Proceedings: Innovations, Quality & Outcomes. 2019;3(3):358-7 Doi: https://doi.org/10.1016/j.mayocpiqo.2019.04.006
- Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. Journal of the American College of Cardiology. 2016 Jul 26;68(4):411-21. Doi: https://doi.org/10.1016/j.jacc.20103.605
- Blankstein R, Stewart GC, McKenna WJ.: Clinical Manifestation and Diagnosis of Cardiac Sarcoidosis. 2018
- Kamangar N, Rohani P, and Shorr AF. Sarcoidosis Clinical Presentation Medscape, https://emedicine.medscape.com/article/301914-clinical#b1 (19 March 2018).
- Alahmadi DH, Alharthi WM, Jifri AT, Alkhabbaz MJ, Alharbi MK, Alruwaili HI, Almughais TJ, Alrehaili MS, Hanif BM, Alzaid FN, Rajh DA. Evaluation of Recent Updates Regarding the Diagnosis and Management of Congenital Heart in Children. Arch. Pharm. Pract. 2019;10(3):29-33.
- Arrhythmia. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/health-topics/arrhythmia
- Doughan A and Williams BR. Cardiac sarcoidosis. Heart 2006;92(2):282–288. http://dx.doi.org/10.1136/hrt.2005.080481
- Koene RJ, Adkisson WO, Benditt DG. Syncope and the risk of sudden cardiac death: Evaluation, management, and prevention. J Arrhythm. 2017;33(6):533-544. https://doi.org/10.1016/j.joa.2017.07.005
- Sekhri V, Sanal S, DeLorenzo LJ, Aronow WS, Maguire GP. Cardiac sarcoidosis: a comprehensive review. Archives of medical science: AMS. 2011 Aug;7(4):546. Doi: https://dx.doi.org/10.5114%2Faoms.2011.24118
- Linder J, Hidayatallah N, Stolerman M, McDonald TV, Marion R, Walsh C, Dolan S. Perceptions of an implantable cardioverter-defibrillator: A qualitative study of families with a history of sudden life-threatening cardiac events and recommendations to improve care. The Einstein journal of biology and medicine: EJBM. 2013;29(1-2):3. https://doi.org/10.23861/ejbm20132929
- Yodogawa K, Seino Y, Ohara T, Takayama H, Katoh T, Mizuno K. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Annals of Noninvasive Electrocardiology. 2011 Apr;16(2):140-7. https://doi.org/10.1111/j.1542-474x.2011.00418.x
- Kroesen VM, Gröschel MI, Martinson N, Zumla A, Maeurer M, van der Werf TS, Vilaplana C. Non-steroidal anti-inflammatory drugs as host-directed therapy for tuberculosis: a systematic review. Frontiers in immunology. 2017 Jun 30;8:772. https://doi.org/10.3389/fimmu.2017.00772
- McKinzie BP, Bullington WM, Mazur JE, Judson MA. Efficacy of short-course, low-dose corticosteroid therapy for acute pulmonary sarcoidosis exacerbations. The American journal of the medical sciences. 2010 Jan 1;339(1):1-4. https://doi.org/10.1097/maj.0b013e3181b97635
- Nagai S, Izumi T. Treatment with methotrexate in patients with sarcoidosis. InSarcoidosis 2013 Mar 13. IntechOpen. https://doi.org/10.5772/55042
- Nunes H, Freynet O, Naggara N, Soussan M, Weinman P, Diebold B, Brillet PY, Valeyre D. Cardiac sarcoidosis. InSeminars in respiratory and critical care medicine 2010 Aug (Vol. 31, No. 04, pp. 428-441). © Thieme Medical Publishers. https://doi.org/10.1055/s-0030-1262211
- Lower EE., Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995;155(8):846-851. https://doi.org/10.1001/archinte.1995.00430080088011
- Webb M, Conway KS, Ishikawa M, Diaz F.: Cardiac Involvement in Sarcoidosis Deaths in Wayne County, Michigan: A 20-Year Retrospective Study. Acad Forensic Pathol. 2018;8(3):718-28. https://doi.org/10.1177/1925362118797744
- Mont L, Pelliccia A, Sharma S, Biffi A, Borjesson M, Terradellas JB, Carré F, Guasch E, Heidbuchel H, Gerche AL, Lampert R. Pre-participation cardiovascular evaluation for athletic participants to prevent sudden death: Position paper from the EHRA and the EACPR, branches of the ESC. Endorsed by APHRS, HRS, and SOLAECE. Ep Europace. 2017 Jan 1;19(1):139-63. https://doi.org/10.1093/europace/euw243
- Sekiguchi M, NUMAO Y, IMAI M, FURUIE T, MIKAMI R. Clinical and histopathological profile of sarcoidosis of the heart and acute idiopathic myocarditis concepts through a study employing endomyocardial biopsy I. sarcoidosis: symposium on secondary myocardial disease. Japanese circulation journal. 1980 May 20;44(4):249-63. https://doi.org/10.1253/jcj.44.249
- Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med 1977;63(1):86-108. https://doi.org/10.1016/0002-9343(77)90145-0
- Tan JL, Sharma SK. Cardiac sarcoidosis presenting with syncope and rapidly progressive atrioventricular block: a case report. Eur Heart J Case Rep. 2018; 2(4):yty103. https://doi.org/10.1093/ehjcr/yty103
- Kopriva P, Griva M, Tüdös Z. Management of cardiac sarcoidosis–A practical guide. Cor et Vasa. 2018; 60(2):e155-64. https://doi.org/10.1016/j.crvasa.2017.05.012
- Darda S, Zughaib ME, Alexander PB, Machado CE, David SW, Saba S. Cardiac sarcoidosis presenting as constrictive pericarditis. Tex Heart Inst J. 2014;41(3):319-323. https://doi.org/10.14503/thij-13-3208
- Chopra A, Kalkanis A, Judson MA. Biomarkers in sarcoidosis. Expert Rev Clin Immunol. 2016;12(11):1191-1208. https://doi.org/10.1080/1744666x.2016.1196135
- Ahmadzai H, Loke WS, Huang S, Herbert C, Wakefield D, Thomas P. Biomarkers in sarcoidosis: a review. Curr. Biomark. Find. 2014; 4:93. https://doi.org/10.2147/cbf.s46196
- Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol. 2017;70(13):1618-36.
- Yazaki Y, Isobe M, Hiroe M, Morimoto SI, Hiramitsu S, Nakano T, Izumi T, Sekiguchi M, Central Japan Heart Study Group. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. The American journal of cardiology. 2001 Nov 1;88(9):1006-10. https://doi.org/10.1016/s0002-9149(01)01978-6
- Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. Journal of the American College of Cardiology. 2005 May 17;45(10):1683-90. https://doi.org/10.1016/j.accreview.2005.08.215
- Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager JH. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999;16(1):75–86.
- Diagnostic standard and guidelines for sarcoidosis [in Japanese]. Jpn J Sarcoidosis Granulomatous Disorder 2007;27:89–102. https://doi.org/10.17996/anc.17-00029
- Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Nielsen JC, Patel AR. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart rhythm. 2014 Jul 1;11(7):1304-23. https://doi.org/10.1016/j.hrthm.2014.03.043
- Baughman RP, Lower EE. Steroids for sarcoidosis: How much and for how long? Respir. Med. 2018; 138:S5-6. https://doi.org/10.1016/j.rmed.2017.12.009
- Iannuzzi MC, MD, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357:2153-2165. https://doi.org/10.1056/nejmra071714
- Valeyre D, Prasse A, Nunes H, et al.: Sarcoidosis. Lancet 2014;383:1155-1167. https://doi.org/10.1016/s0140-6736(13)60680-7
- Demeter SL. Myocardial Sarcoidosis Unresponsive to Steroids. Chest 1988;94(1):202-203. https://doi.org/10.1378/chest.94.1.202
- Swigris JJ, Olson AL, Huie TJ, Fernandez-Perez ER, Solomon J, Sprunger D, Brown KK. Sarcoidosis-related mortality in the United States from 1988 to 2007. American journal of respiratory and critical care medicine. 2011 Jun 1;183(11):1524-30. https://doi.org/10.1164/rccm.201010-1679oc
- Yatsynovich Y, Dittoe N, Petrov M, Maroz N. Cardiac sarcoidosis: a review of contemporary challenges in diagnosis and treatment. Am J Med Sci. 2018;355(2):113-25. https://doi.org/10.1016/j.amjms.2017.08.009.
