Toxicological Evaluation of Nelumbo Nucifera Fruit Ethanol Extract
Muhammad Ali Rajput1, 2*, Tabassum Zehra3, Shahid Zafar4, Gunesh Kumar5, Fizzah Ali3, Nadia Aslam6
1Department of Pharmacology, Multan Medical & Dental College, Multan, Pakistan.
2 Department of Pharmacology, University of Health Sciences, Lahore, Pakistan.
3 Department of Pharmacology, Liaquat National Medical College, Karachi, Pakistan.
4 Department of Pathology, Liaquat College of Medicine & Dentistry, Karachi, Pakistan.
5Department of Pharmacology, Liaquat University of Medical & Health Sciences, Jamshoro, Sindh, Pakistan.
6 Department of Forensic Medicine, Liaquat University of Medical & Health Sciences, Jamshoro, Sindh, Pakistan.
*Email: drmuhammadali2016 @ gmail.com
ABSTRACT
Nelumbo nucifera fruit (NNF) is frequently used for the treatment of many diseases in Asian countries without proper scientific evidence of its safety. The purpose of this study was to determine the toxicological effects of NNF. Toxicity study was conducted on 28 male Wister rats weighing 180-230 g that were allocated equally to 4 treatment groups; a control and 3 test groups. Parameters assessed were clinical signs, body weight, hematology, blood biochemistry and histopathology after administration of NNF to rats for 13 weeks. No major toxicity was revealed throughout the study, though some biochemical changes were observed in hepatic and renal tissues but these changes did not correspond with histopathology findings. There was no mortality and evidence of systemic toxicity following 13 weeks administration of NNF. Hematology and blood biochemistry did not reveal any toxicity at any dose; however, histopathological evaluation of hepatic tissues of few animals treated with 200 mg/kg showed areas of necrosis at lesser extent in few animals after 13 weeks exposure of fruit. Histopathology of renal tissues of group treated with 200 mg/kg revealed areas of moderate tubular disruption and few foci of tubular necrosis. Although only few adverse effects were observed but NNF administration if necessary for a prolonged period, then it may be used in a dose rage of 50-100 mg/kg in order to avoid intractable effects. Additional studies are required to clinically evaluate the safety profile of NNF.
Key words: Nelumbo nucifera, hematology, biochemical, histopathological, tubular necrosis, toxicity.
INTRODUCTION
The use of herbal drugs is becoming progressively more popular as they are supposed to be natural, advantageous and lack unwanted effects. Mostly the plant-derived drugs are taken randomly by local population for the treatment of various diseases without having adequate information regarding its usefulness. Hence, for proper guidance of the general population, especially users of natural products, there is a need to scientifically prove the effectiveness of medicinal plants [1].
Nelumbo nucifera, a plant from Nymphaeaceae family is commonly cultivated in the hot and humid climatic zones of Thailand, Pakistan, India and China [2]. Its fruit contains seeds plus pods (lotus bulbs). The green colored pods offer add-on to the seeds, which are hard in nature, ovoid to roundish in shape i.e. 1.5 cm×1.0 cm broad and long and are organized in whorls [3]. The seeds are the edible portion and have to be skinned separately before eating [4].
Its seeds are a wonderful source of protein, fat, asparagines, unsaturated fatty acids, carbohydrates, gallic acid and isoquininolinol. They also contain ample amount of various minerals such as potassium, magnesium, calcium, sodium, iron, chromium, manganese, copper and zinc [2, 5, 6].
A recently conducted study on NNF pods has shown the existence of numerous active bioactive principles including flavonoids, alkaloids, saponins, terpenoids and tannins. Procyanidin (alkaloid) was also squeezed from NNF pods [2].
Habitually, the fruits are used as a healthy component of Asian cuisine and also as a traditional cure of various ailments e.g. hypertension, palpitation, arrhythmia, fever, pain, inflammation, sleep disorders, diarrhea, leucorrhoea, bad breath and bleeding disorders [7, 8]. It equally has anxiolytic, antidepressant and antiepileptic effects [9, 10]. Though recent research work revealed LD50 value of NNF higher than 5g/kg, still very inadequate literature was obtainable regarding its toxicological effects after prolong use. The purpose of this study was to determine the toxicological effects of NNF after administration of NNF to rats for 13 weeks.
MATERIALS AND METHODS
This research project was conducted using the laboratory and workshop facilities of Research Institute of Pharmaceutical Sciences and HEJ Research Institute of Chemistry, UOK following approval of synopsis and after getting permission for the use of laboratory animals (BASR/02149/Pharm, dated 30th December 2014).
Animals
Male Wister rats (28) weighing 180-230 g were used for this toxicity study in line with protocol from NACLAR (National Advisory Committee for Laboratory Animal Research) and NIH (National Institute of Health) [11, 12]. The animals were housed in polycarbonate cages with cage enrichment and were allowed to acclimatize for three weeks before the start of the experiment. The temperature was kept at about 25°C with relative humidity of 50 to 60% in an alternating twelve-hour light and dark cycle. Each rat was provided access to normal diet and water. The animals were carried to the laboratory about an hour prior to the initiation of the experiments.
Chemicals/Extract
After obtaining fruits from domestic fruit bazaar of Hyderabad, Pakistan in September 2016, they were initially presented to Pharmacognosy Department, UOK for identification and validation and afterwards receipt no NNF-03 was deposited in the same department. Crude extract was prepared through cold extraction procedure [13, 14]. 6 kg fruits were initially rinsed with tap water and the seeds were separated from the pods manually The seeds have high contents of water that’s why they need to be chopped first then left for 6 days for drying out in shade. The dried material obtained was so thick so that needed to be grounded into fine powder. In contrast, the pods were chopped once only and were allowed to dry in shade for just 03 days. The dried pod material took a coarse powder form. So, for better separation and collection of NNF constituents (secondary metabolites), they needed to be chopped and dried separately before soaking up together in ethanol (98%) for thirty days with occasional shaking. Afterwards, it was sieved using separator (Whatman No. 1). Later, it was evaporated using rotary machine under condensed pressure at 40°C to 45°C. The condensed material was freeze dried in a freeze dryer at -30°C. The material gained was preserved at -20°C until further use in doses of 50, 100 and 200mg/kg orally. The ultimate amount of the extract acquired was 400 g of dry weight.
2% tragacanth gum in powder form was acquired from Merck which was consumed to make suspensions of 3 different doses of test drug i.e. NNF 50, 100 & 200 mg/kg. It was given to the control group as placebo in the dose of 10ml/kg orally. 100ml of warm distilled water was added in 2 g tragacanth gum to make 2% suspension. At each occasion, new suspensions were prepared for dosing [15, 16].
Experimental design
Sub-chronic toxicity study was conducted according to the method described by Niho et al., 2001 with slight modification [17].
Male Wister rats (28) weighing 180-230 g were used and were divided equally into four treatment groups; a control and 3 test groups. The gross examination of rats was carried out at every week following administration of drugs precisely noticing average variation in weight, hair loss, ulceration, lacking interest in food, loss of activity, hematuria, lacrimation, salivation, diarrhea, vomiting, edema, tremor, muscle tone and aggressive actions. Weekly body weight was also noted. Towards the end of dosing, the animals were sacrificed under anesthetic condition using ether and autopsy was executed by random selection and then samples for hematology and blood biochemistry were collected for analysis. Hepatic and renal tissues were also assessed for histopathology.
Hematology
7ml blood sample was collected from each animal through cardiac puncture towards the end of dosing period of 90 days. Blood samples were immediately centrifuged (Heraeus, Christ Labofuge A) at 4000 rpm for 8 minutes to collect serum, which were than analyzed within 3 hours on Vita Lab eclipse automatic analyzer (Merck) using standard reagent kits obtained from Merck. Blood samples were collected under 10% EDTA at 7.2pH and then hemoglobin concentration, red blood cells, white blood cells and platelet counts were measured on Huma count plus a fully automated hematology analyzer (Human Germany) [18].
Blood biochemistry
Renal parameters (Total Proteins, Urea & Creatinine)
Serum protein was analyzed according to biuret technique using Vita Lab Eclipse in which proteins and peptides produce a violet color complex with copper ions in presence of sodium hydroxide [19]. Sample absorbance was checked against reagent blank at 546 nm within 60 min. on Vitalab that directly gives the total protein concentration in g/dl.
Urea was determined enzymatically. The reduction in NADH absorbance per unit time is proportional to the Urea concentration. Absorbance was measured at 340 nm in Vita lab that directly gives the urea levels in mg/dl. Serum creatinine activity was evaluated by a colorimetric reaction known as Jaffe reaction [20]. Creatinine forms a yellow-orange compound in alkaline solution with picric acid. The concentration of the dye over a certain reaction time is a measure of the creatinine concentration [21]. Absorbance of sample and standard were read at 492 nm on Vita lab that directly gives serum creatinine activity in mg/dl.
Hepatic parameters (Alkaline phosphatase, Alanine transaminase, Aspartate transaminase)
The concentration of enzyme alkaline phosphate in serum was assessed photometrically, with the help of Ecoline 125 AP supplied by Merck. The rate of increase in 4- nitrophenolate was evaluated photometrically, which was directly proportional to the activity of enzyme alkaline phosphatase present in sample serum [22]. The escalation in absorbance was calculated at 405nm every minute for 3 min, on Vita lab, that directly gives the concentration of enzyme alkaline phosphatase in U/l.
The level of enzyme Alanine transaminase (ALT) in serum was assessed by utilizing Ecoline 125, ALAT Tris of Merck. The rate of NADH utilization was measured photometrically, which was directly proportional to the ALT activity in the sample. The reduction in absorbance was measured at 340 nm every minute for 3 minute on Vita Lab that directly gives the level of the enzyme in U/l.
The level of enzyme Aspartate transaminase (AST) in serum was assessed by utilizing Ecoline 125, ASAT supplied by Merck. The rate of NADH consumption was measured photometrically, which is directly proportional to the AST activity in the sample. The reduction in absorbance was measured at 340 nm, every minute for 3 minutes on Vita lab that directly gives the level of the enzyme AST in U/l.
Histopathology
Specimens of hepatic and renal tissues were conserved in 10% buffered formalin. Appropriate blocks of these organs were taken, fixed and the sections were cut for microscopic examination.
Representative sections from various regions of these organs were sliced after extrication of the fat from individual organs. The blocks were processed in an automatic tissue processor (Gilford 101 system) and after that embedded in paraffin at 56º C in hot air oven. These paraffin embedded tissues were then sectioned with microtome (3 to 4 micron thickness) and stained with hematoxylin and eosin stain and were evaluated histologically with light microscope under lens 10x & 40x.
Statistical analysis
The data obtained through experiments were used to determine mean and standard error to the mean using two sample student T- test and values of P less than 0.05 were reflected as significant and P less than 0.005 as extremely significant. All numerical methods were accomplished with SPSS software version 20 [23].
RESULTS
Physical examination
The animals in any group did not reveal any significant toxicities and gross anomalies during the total period of study, i.e. 13 weeks. There were no hair loss, skin ulceration, loss of activity, diarrhea, salivation, vomiting, hematuria, edema, tremor and aggressive behavior. The data also showed no significant difference in the mean weight variation.
Hematology/Biochemistry
Table 1 reveales the comparison of hematological parameters i.e. red blood cell count, white blood cell count, platelet count and hemoglobin following 90 days oral administration of NNF in three doses i.e. 50, 100 and 200 mg/kg in different groups against control. There were no significant difference noted in any of the parameters at any dose in comparison to the control.
Table 2 shows the outcome of NNF following its oral administration in three dosages; 50, 100 and 200 mg/kg against control on biochemical parameters i.e. total protein, urea, creatinine, alkaline phosphates, ALT and AST. No significant biochemical changes were observed and recorded in the groups treated with 50 and 100 mg/kg dosage of NNF when compared to the control, but the group treated with NNF 200 mg/kg showed notable increase in blood creatinine and ALT levels as compared to the control, though the changes were in normal physiological limits.
Table 1: Effect of different doses of NNF on hematological parameters in rats
|
Parameters |
Control |
NNF 50 mg/kg |
NNF 100 mg/kg |
NNF 200 mg/kg |
|
Red blood cells (×106/µl) |
7.27±0.01 |
6.94±0.19 |
6.76±0.22 |
6.77±0.21 |
|
White blood cells (×103/µl) |
6.47±0.09 |
6.31±0.08 |
6.40±0.082 |
6.68±0.05 |
|
Platelet (×103/µl) |
371.11±0.51 |
369.63±0.88 |
369.34±0.95 |
370.60±0.64 |
|
Hemoglobin (g/dl) |
9.50±0.19 |
9.77±0.20 |
9.80±0.20 |
9.11±0.04 |
n=7
The expressions were calculated by taking mean ± standard error to the mean.
Table 2: Effect of different doses of NNF on biochemical parameters in rats
|
Parameters |
Control |
NNF 50 mg/kg |
NNF 100 mg/kg |
NNF 200 mg/kg |
|
Total Protein g/dl |
6.6±0.08 |
6.3±0.1 |
6.4±0.1 |
6.8±0.1 |
|
Urea mg/dl |
29.0±1.7 |
25.5±1.2 |
27.3±0.6 |
26.0±1.9 |
|
Creatinine mg/dl |
0.7±0.03 |
0.71±0.0 |
0.7±0.02 |
0.8±0.03* |
|
AST U/l |
18.5±1.1 |
22.1±2.2 |
19.8±1.5 |
19.14±1.2 |
|
ALT U/l |
22.8±1.7 |
21.3±0.6 |
24.7±1.4 |
28.5±1.5* |
|
ALP U/l |
55.8±3.3 |
62.8±1.3 |
64.7±6.3 |
63.8±2.6 |
n=7
The expressions were calculated by taking mean ± standard error to the mean.
*p value less than 0.005 was counted as significant in comparison to the control.
Histopathology
Gross examination of vital organs e.g. liver and kidneys did not reveal any macroscopic changes in any group.
The rats provided with 50 and 100 mg/kg dose showed focal areas of ballooning (Vacuolar) degeneration and mild inflammatory changes around the portal tract (figure 1&2). The animals treated with 200 mg/kg in addition to mild areas of vacuolar degeneration and moderate inflammatory changes also showed areas of necrosis at lesser extent (figure 3&4). However, there were no microscopic changes in liver architecture, hepatocytes and central vein in the control group (Figure 5).
The rats treated with 50 mg/kg dose of NNF showed focal areas of tubular derangement (Figure 6). The rats given 100 mg/kg dose of NNF showed few areas of tubular vacuolization (Figure 7). The animals treated with 200 mg/kg revealed areas of moderate tubular disruption and few foci of tubular necrosis along with moderate interstitial edema (Figures 8&9). No microscopic changes were observed in renal tissues of the control group (Figure 10).
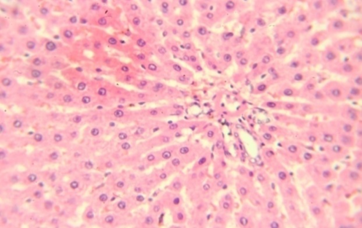
Figure 1: Hepatic tissue showing mild inflammation and vacuolar degeneration 40x
Figure 2: Hepatic tissue showing ballooning of hepatocytes10x
Figure 3: Hepatic tissue showing inflammatory changes around portal tract 10x
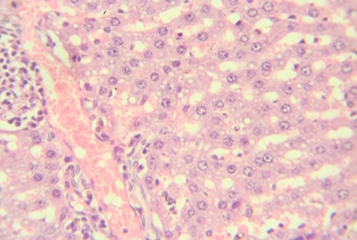
Figure 4: Hepatic tissue showing few areas of necrosis with ballooning 40x
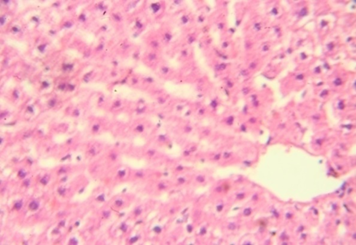
Figure 5: Normal hepatic tissue showing intact hepatocytes 40x
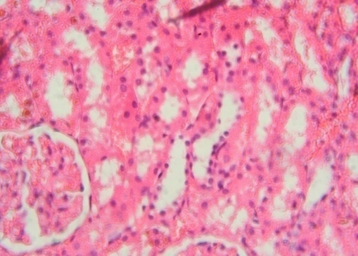
Figure 6: Renal tissue revealing few foci of tubular derangement 40x
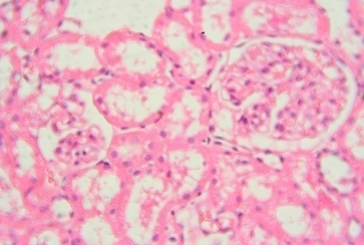
Figure 7: Renal tissue showing foci of tubular vacuolization 40x
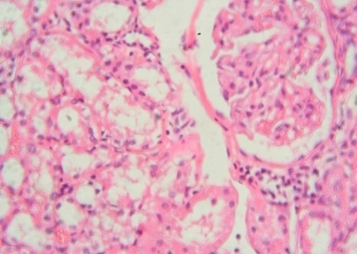
Figure 8: Renal tissue showing moderate tubular disruption and necrosis 40x
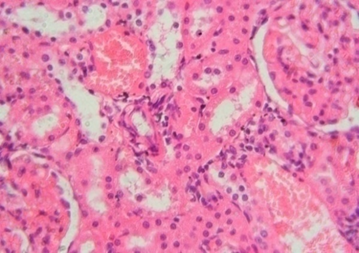
Figure 9: Renal tissue showing congestion, tubular disruption and interstitial edema 40x
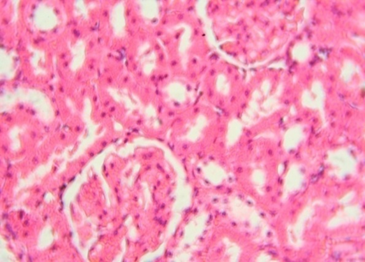
Figure 10: Normal renal tissue 40x
DISCUSSION
The current study demonstrated the safety profile of NNF given as a dietary admixture to the rats following exposure for 13 weeks as no such sub-chronic toxicity assessment of NNF was performed previously. Hematological parameters were not altered significantly with any dose of NNF extract as compared to the control. In addition to this, biochemical changes were also not exposed in the groups treated with 50 and 100 mg/kg NNF but significant increase was noted in ALT and creatinine of the group treated with 200 mg/kg of NNF as compared to the control; however, the rise in both parameters was in a normal physiological limit.
Certain enzymes, not originally produced in serum, but in fact leak in to the serum at some point of tissue damage are a valuable source in clinical diagnosis. They are informative on the effect and nature of pathological loss to any tissue. ALT & AST, sensitive markers of hepatocellular loss, can provide a quantitative assessment of certain degree of damage to liver [24, 25].
Generally, ALT levels are constantly higher than AST and the reason for this is that body cells generate little higher ALT in contrast to AST [26]. Approximately 80% of ALT is originated in mitochondria while AST solely resides in cytoplasm; as a result ALT emerges in greater concentrations from various tissues such as liver, kidneys, heart and pancreas and is released gradually as compared to AST. Though AST is confined primarily to cytosol of hepatocytes but is believed to be a more sensitive indicator of hepatocellular injury than ALT [27].
Similarly urea and creatinine are sensitive indicators of renal function [28]. Damage to glomeruli causes substantial decline in GFR and may raise the levels of both markers in the blood which subsequently results in chronic renal failure [29].
In the present study, gross inspection of hepatic and renal tissues did not show any significant macroscopic changes. Microscopic examination of hepatic and renal tissues also showed no substantial changes in the control and extract treated groups of 50 and 100 mg/kg of NNF. In addition to this, most of the hepatic and renal tissue sections of animals treated with NNF 200 mg/kg did not show any noteworthy histopathological changes; however, few animals which were treated with 200 mg/kg of NNF showed mild areas of vacuolar degeneration and moderate inflammatory changes as showed in Figures 3 and 4. Likewise, few animals treated with 200 mg/kg of fruit revealed areas of moderate tubular disruption and few foci of tubular necrosis along with moderate interstitial edema as revealed in Figures 8 and 9. Although these changes in general were reversible and were noted in only few animals, most of the animals treated with NNF 200 mg/kg were found normal.
CONCLUSION
The present study revealed that NNF 200 mg/kg can be used for short term without any danger of side effects but if required for a prolonged period, then it may be used in a dose rage of 50-100 mg/kg in order to avoid intractable effects. Clinical studies are required to further appraise the biological implications of observed mild changes in hepatic and renal tissue sections.
ACKNOWLEDGMENT
The authors are thankful to the Chairman Pharmacognosy Department, Karachi University for identification and authentication of NNF and also appreciate the support of Director, International Center for Chemical and Biological Sciences (ICCBS) for permitting us to use their facilities to complete this research work.
Conflict of Interest
The authors declare no conflict of interest.
Funding Statement
No funding was acquired from any source/organization.
REFERENCES
- Agbaje EO, Adeneye AA, Daramola AO. Biochemical and toxicological studies of aqueous extract of Syzigium aromaticum L. Merr. & Perry Myrtaceae in rodents. Afr J Trad CAM, 2009, 6:3, 241-54.
- Mukherjee PK, Mukherjee D, Maji AK, Rai S, Mukherjee PK, Saha K et al. The sacred lotus (Nelumbo nucifera) phytochemical and therapeutic profile. Journal of Pharmacy and Pharmacology, 2009; 61: 407–42 PMID:19298686
- Sridhar KR, Bhat R. Lotus: a potential nutraceutical source. J Agri Technol, 2007; 3: 143–155.
- Carlo FM, Masami Y, Yoshiaki K, Ganesh KA, Randeep Rakwal. Lotus – A Source of Food and Medicine: Current Status and Future Perspectives in Context of the Seed Proteomics. IJLS, 2013, 7, 1-5.
- Indrayan AK, Sharma S, Durgapal D, Kumar N, Kumar M. Determination of nutritive value and analysis of mineral elements for some medicinally valued plants from Uttaranchal. Curr Sci, 2005, 89: 1252–5
- Pal I, Dey P. A review on lotus (Nelumbo nucifera) seed. International Journal of Science and Research, 2015; 4(7): 1659-166
- Chopra RN, Chopra IC, Nayar SL. Glossary of Indian Medicinal Plants. New Delhi: Council of Scientific and Industrial Research 1996, 174.
- Varshney CK, Rzoska J. Aquatic weeds in South East Asia, 1sted. New Delhi: Springer 1976, 39.
- Rajput MA, Khan RA. Phytochemical screening, acute toxicity, anxiolytic and antidepressant activities of Nelumbo nucifera fruit. Metab Brain Dis, 2017; 32(3): 743-74 DOI 10.1007/s11011-017-9963-x
- Rajput MA, Khan RA, Assad T: Anti-epileptic activity of Nelumbo nucifera fruit. Metab Brain Dis, 2017; 32, 1883-1887. DOI: 1007/s11011-017-0064-7.
- National Advisory Committee for Laboratory Animal Research. Guidelines on the care and use of animals for scientific purposes. 2004, p. 24.
- National Institute of Health Guidelines for Care and Use of Laboratory Animals in Biomedical Research. Guide for the care and use of laboratory animals. Prepublication draft. 8thed. The National Academies Press Washington, D.C; 2010, 6, 47.
- Hossain MS, Ahmed M, Islam A. Hypolipidemic and hepatoprotective effects of different fractions of ethanolic extract of immature leaves of Mangifera indica Linn in alloxan induced diabetic rats. Journal of Pharmaceutical Sciences and Research, 2010, 1, 132-38.
- Assad T, Khan RA, Rajput MA. Effect of Trigonella foenum-graecum Linn seeds methanol extract on learning and memory. Metab. Brain Dis, 2018; 33:1275-1280. doi: 10.1007/s11011-018-0235-1
- Madhu A, Keerthi PHV, Singh J, Shivalinge GKP. To evaluate the anti-epileptic activity of aqueous root extract of Hemidesmus indicus in rats. Arch. Pharm. Sci. Res 2009; 1(1), 43-7.
- Khan RA, Rajput MA, Assad T. Effect of Nelumbo nucifera Fruit on Scopolamine Induced Memory deficits and on Motor Coordination. Metab Brain Dis, 2018; 34(1):87-92. https://doi.org/10.1007/s11011-018-0324-1. Epub 2018 Oct 1.
- Niho N, Shibutani M, Tamura T, Toyoda K, Uneyama C, Takahashi N. Subchronic toxicity study of gallic acid by oral administration in F344 rats. Food and Chemical Toxicology, 2001; 39: 1063–70.
- Ahmed S, Khan RA, Feroz Z. Assessment of sub-chronic, hematological and histopathological toxicities of a herbal combination. Pak J Pharm Sci, 2015; 28(6):2153-60.
- Thomas L. Clinical Laboratory Diagnostics. 1sted. TH Books Verlagsgesells chaft. Frankfurt 1998; p 136-46.
- Seeling HP, Wuest H. Creatinine determination by the Jaffe reaction. Aerztl. Lab 1969; 15(1): 34-39.
- Bartels H, Bohmer M, Heierli C. Serum creatinine determination without protein precipitation. Clin. Chim. Acta, 1972; 37: 193-97.
- Recommendation of the German Society of Clinical Chemistry. Standardization of methods for measurement of enzymatic activities in biological fluids. Z Klin Chem Klin Biochem, 1972; 10:182-92.
- Walpole RE. Introduction of Statistics. Third Edition, Macmillan Publishing Company, Inc., New York, 1982, p. 247-304.
- Will's DE. Biochemical basis of medicine. Bristol: John Wright and Sons Ltd 1985; 1:45–66.
- Al-Habori M, Al-Aghbari A, Al-Mamary M, Baker M. Toxicological evaluation of Catha edulis leaves: A long term feeding experiment in animals. J Ethnopharmacol 2002; 83:209–17.
- Mayne PD. Clinical Chemistry in Diagnosis and treatment, 6thed (International Students Edition). Arnold London/Oxford University Press Inc., New York 1996.
- Saritha V, Anilakumar KR. Toxicological evaluation of methanol extract of Aloe vera in rats. Int. J. Pharmaceut. Biomed; Res 2010; 1: 142-49.
- Odoula T, Adeniyl FA, Bello IS, Subair HG. Toxicity studies on an unripe Carica papaya aqueous extract: Biochemical and haematological effects in Wister albino rats. J Med Plants Res 2007; 1:1–4.
- Ramakrishnan S, Swami R. Text Book of Clinical Biochemistry and Immunology; Vol.3, Chennai: T.R Publications 1995; p 245-75.
