Cardiotherapeutic Effect of Naringin and Hesperidin via Anti-Inflammatory and Antioxidant Effect in Experimental Model
Hanan A. Al-Sefri1*, Widad M. Al-Bishri2, Amal M. Hamza2, 3
1 Biochemistry Department, Faculty of Science, King AbdulazizUniversity, Jeddah, Saudi Arabia.
2University of Jeddah, College of Science, Department of Biochemistry, Jeddah, Saudi Arabia.
3Biochemistry and Nutrition Department, Faculty of Women, Ain ShamsUniversity, Egypt.
*Email: hanan.alsefri91 @ gmail.com
ABSTRACT
Myocardial infarction (MI) is the main cause of heart failure and has a high prevalence and mortality and a great impact on social life. Naringin (Nar) and hesperidin (Hes) are citrus flavonoids, with powerful biological properties. The present study aimed to show the ameliorative effect of naringin and hesperidin and their combinations on improvement MI induced experimentally. Fifty rats were divided into five groups (ten rats each) as follow: Group 1: Healthy control group, Group 2: cardiac dysfunction (CD) induced group by adriamycin (ADR), Group 3: (CD + Nar) induced rats treated orally with naringin, Group 4: (CD + Hes) induced rats treated orally with hesperidin, Group 5: (CD +Nar+ Hes) induced group were treated orally with combination of naringin and hesperidin. Biochemical results revealed significant elevation of markers in groups treated with naringin, hesperidin and their combinations. Our results showed a significant increase in ox-LDL and MDA accompanied by a decrease in SOD in the CD-induced group. Combinations of treatment with naringin and hesperidin showed great improvement effects in all biochemical markers under investigation. The biochemical aspects have also been confirmed by histological findings. In conclusion: outcomes of the present study confirmed that treatment by either naringin, hesperidin or their combinations of naringin and hesperidin have a promising effective rate in ameliorating CD-induced experimentally.
Key words: Naringin, Hesperidin, Cardiacdysfunction, Myocardial infarction, Cardiac biomarkers, Inflammation, Oxidative stress.
INTRODUCTION
Cardiovascular disease (CVDs) is a chronic disease of the heart and the arteries. The term is commonly used to refer to the diseases related to arteriosclerosis and other arterial diseases [1]. The mortality due to heart diseases increases every year and has exceeded that accrued to cancer [2, 3]. Coronary and heart diseases are a major cause of mortality in limited-resource settings [4, 5]. The risk factors for atherosclerosis can be targeted to lower cardiovascular diseases [6]. Urbanization in Saudi Arabia has increased recently, doubling of the people living in an urban setting [7]. The high rate of urbanization has led to the lifestyle of the majority of people living in town and a consequent increase in the morbidity of cardiovascular diseases. However, the data from the National preventive health system show a lower prevalence of cardiovascular diseases in Saudi Arabia [8]. For a long time, the antitumor drug Adriamycin has been used to treat cancer including lymphomas, leukemia, solid tumors, and soft-tissue sarcomas [9]. Unfortunately, adriamycin has severe, irreversible and dose-dependent cardiotoxicity [10]. However, despite long term use of Adriamycin to treat cancers, this drug has been associated with toxicity resulting in mitochondria impairment, oxidative stress, an overload of calcium and apoptosis [11, 12]. The drug results in the production of reactive oxygen species (ROS) that cause cardiomyocyte apoptosis [13]. Flavonoids from citrus fruits with polyphenolic compounds have helped treat metabolic dysregulation. Some of the flavonoids in citrus fruits include naringenin, hesperidin, nobiletin, and tangeretin. Besides, epidemiological studies have emerged, demonstrating the medical value of the flavonoids to reduce cardiovascular diseases. Among the biological properties of the flavonoids, antihypertension, insulin sensitization, lipid-lowering and anti-inflammatory activities are the most evident based on previous studies [14]. The active compound Naringin (4′,5,7-trihydroxyflavone 7-rhamnoglucoside), in grape and citrus fruits [15], have anti-inflammatory and cardio-protective activities such as lowering of blood glucose, increased insulin sensitivity and loweringcholesterollevels [16]. Hesperidin(C28H34O15) is a flavanone glycoside, considered as a member of citrus flavonoids in oranges [17]. Hesperidiniscommonlyused as a supplement known to reduce the effects of CDVs, antidiabetic effects, and reducing blood coagulation. The hesperidin is commonly used to reduce hypertension, and myocardial infarction [18]. Despite decades of research on the biological effects of flavonoids, there are no studies that have been performed on the combinatory effects of naringin and hesperidin on cardiac dysfunction. The current study opens up opportunities to come up with more effective therapeutic natural compounds effective against CVDs.
MATERIALS AND METHODS:
Chemicals:
Kits used in this study were purchased from NOVA (Beijing, China). Naringin was sourced from Swanson Health Products (Fargo, ND 58104 United States), and Hesperidin was sourced from Douglas Laboratories (Pittsburgh, PA 15205 United States) whileAdriamycinwasobtainedfrom King AbdulazizUniversityHospitalpharmacy (Jeddah, Saudi Arabia).
Animals:
Model animals used in this study included 50 male albino rats weighing 120-130g. The animals were obtained from the Animal House Colony of King Fahd Medical Research Center. The animals were acclimatized for one week before the experiment commenced. The mice were maintained at a standard housing facility at the King Fahd Medical Research Center Animal Facility Breeding Colony. The mice were maintained on laboratory chow and water ad libitum.
Experimental Design
The experiment was performed by dividing the 50 rats into five groups (ten rats each) and treated as follows. The first group was the control group. This group was treated with 1 ml of oral saline daily. Group 2 was the cardiac dysfunction group, treated with Adriamycin in one dose of (10 mg/Kg bw) [19]. The rest of the groups (3-5) were treated with either naringin, hesperidin and their combination after CD induction in a dose of 20 mg/kg bw for naringin [20] and a dose of 50 mg/kg bw for hesperidin for 30 days [21].
BiochemicalAnalysis and Histopathological
After 45 weeks, the experimental animals were starved overnight and anesthetized. Blood specimens were drawn using capillary micro-tubes. Blood was centrifuged at 3000 rpm for 15 minutes to obtain plasma. Heart-type fatty acid-binding protein (FABP3), Myosin light-chain kinase I (MYLK), Troponins I (cTn-I), Troponins T (cTn-T), creatine kinase MB fraction (CK-MB), lactate dehydrogenase(LDH), B-type Natriuretic Peptide (BNP), C- reactive protein (CRP), Tumor necrosis factor-alpha (TNF-α), OxidizedLDLs (ox-LDL) were determined using ELISA Immunoassayfollowing the manufacturers protocols. Additionally, other markers, including the Malondialdehyde (MDA) and Superoxide dismutase (SOD), aspartate aminotransferase (AST), were estimated in plasma using the spectrophotometric approach. Histopathologicalstudieswereperformed on heart tissues. The sliced tissues were fixed in 10% formalin and sections prepared in paraffin blocks. Staining was performed using hematoxylin and eosin after dewaxing [22].
Statistical Analysis
The values of experimental groups were compared with the values of individual rats. The results were presented as mean ±SE. The analysis of variance was used to compare groups (ONE-WAY ANOVA). All analysis was performed in Statistical Package for the Social Science (SPSS) program (ANOVA), and results were considered statistically significant at p ≤ 0.05.
RESULTS:
Results from the table (1) revealed a significant elevation in cardiac myocyte injurybiomarkersincludingcTn-I, cTn-T, FABP3 and MYLK in the CD group as compared to the healthy control group (P ≤ 0.05). Treatment with either naringin, hesperidin, and their combination showed significant regression in these biomarkers (P ≤ 0.05).
Table (2) showed a significant increase in the CD group in cardiac damage biomarkers (CK-MB, LDH, and AST) as compared to a healthy control group (P ≤ 0.05). Naringin, hesperidin and their combinations groups treatment showed significant regression in these biomarkers (P ≤ 0.05). Exceptedthatis no significant change in CK-MB and AST in combinations groups treatment as compared to the CD group and no significant change in LDH in the hesperidin treated group as compared to the CD group.
In the CD group, a BNP was enhanced as compared to a healthy control group (P≤ 0.05). Treated groups including naringin, hesperidin and their combinations showed a significant decline in BNP level (P ≤ 0.05).
Moreover, the levels of inflammatory markers including CRP and TNF-α in the CD group were elevated as compared to a healthy control group (P ≤ 0.05). All treated groups naringin, hesperidin, and their combinations found a significant regression in these biomarkers (P ≤ 0.05).
We found a significant increase in oxidative stress biomarkers including (ox-LDL, SOD and MDA) in the CD group as compared to the healthy control group (P ≤ 0.05). While, the treatment either with naringin, hesperidin and their combinations showed a significant decrease in these biomarkers (P ≤ 0.05). Also, no significant change in ox-LDL in either hesperidin and combination-treated groups as compared to the CD group.
Our resultsdemonstrated a positive significant correlation betweenheart-type fatty-acid-binding protein and troponins I (r= 0.800, P=0.0001) and troponins T (r= 0.862, P=0.0001) (Figure 1). Also, therewas a positive correlationbetweenmyosin light-chain kinase I and troponins I (r= 0.647, P=0.0001) and troponins T (r= 0.796, P=0.0001) (Figure2). As well as, a positive correlation found between heart-type fatty-acid-binding protein and myosin light-chainkinase I (r= 0.807, P= 0.0001) (Figure 3).
The results in figure (4) demonstrated a positive correlation between heart-type fatty acid-binding protein and creatine kinase MB fraction (r= 0.702, P=0.0001) and aspartate aminotransferase (r=0.660, P=0.0001). Histopathological results are shown in figure (5) (A-E).
Table 1: Effect of naringin, hesperidin, and their combinations on cardiac myocyte injury biomarkers in all studied groups.
|
Groups Parameters |
Control |
CD |
CD + Nar |
CD + Hes |
CD + Nar + Hes |
|
cTn-1 (pg/ml) |
14.97±1.02 |
95.91±3.24a |
22.46±3.35a,b |
19.00±2.20b |
16.19±0.78b |
|
cTn-T (pg/ml) |
58.41±3.94 |
302.20±1.77a |
85.39±2.57a,b |
176.59±5.25a,b,c |
82.23±4.77a,b,d |
|
FABP3(ng/ml) |
0.72±0.15 |
2.42±0.07a |
1.21±0.10a,b |
1.35±0.17a,b |
0.77±0.08b,c,d |
|
MYLK(pg/ml) |
66.99±4.13 |
207.02±19.14a |
137.47±1.69a,b |
149.54±15.27a,b |
70.48±3.52b,c,d |
Data are expressed as mean+/- standard error; percentage change versus control. a: significance versus control; b: significance versus CD; c: significance versus CD + Nar; d: significance versus CD + Hes.
Table 2: Effect of naringin, hesperidin, and their combinations on cardiac damage biomarkers in all studied groups.
|
Groups Parameters |
Control |
CD |
CD + Nar |
CD + Hes |
CD + Nar + Hes |
|
CK-MB (ng/ml) |
0.40±0.03 |
1.43±0.23a |
0.63±0.10b |
0.74±0.03a,b |
0.42±0.03c,d |
|
LDH (ng/ml) |
2.06±0.09 |
5.00±0.64a |
3.43±0.33a,b |
4.21±0.36a |
3.72±0.30a,b |
|
AST (U/ml) |
52.46±0.40 |
57.78±0.45a |
54.28±0.90a,b |
53.63±0.73b |
52.92±0.65a |
Data are expressed as mean+/- standard error ; percentage change versus control. a: significance versus control; b: significance versus CD; c: significance versus CD + Nar; d: significance versus CD + Hes.
Table 3: Effect of naringin, hesperidin, and their combinations on Brain Natriuretic Peptide in all studied groups.
|
Groups Parameters |
Control |
CD |
CD + Nar |
CD + Hes |
CD + Nar + Hes |
|
BNP (pg/ml) |
93.76±2.00 |
400.27±38.91a |
178.81±20.75a,b |
256.68±30.96a,b |
202.04±32.35a,b |
Data are expressed as mean+/- standard errorpercentage change versus control. a: significance versus control; b: significance versus CD; c: significance versus CD + Nar; d: significance versus CD + Hes.
Table 4: Effect of naringin, hesperidin, and their combinations on inflammatory markers in all studied groups.
|
Groups Parameters |
Control |
CD |
CD + Nar |
CD + Hes |
CD + Nar + Hes |
|
CRP (ng/ml) |
3.59±0.30 |
7.74±0.13a |
4.85±0.39a,b |
5.10±0.21a,b |
5.31±0.17a,b |
|
TNF-α (pg/ml) |
96.76±7.35 |
138.60±0.68a |
117.14±0.90a,b |
122.41±1.28a,b |
101.55±1.76b,c,d |
Data are expressed as mean+/- standard error; percentage change versus control. a: significance versus control; b: significance versus CD; c: significance versus CD + Nar; d: significance versus CD + Hes.
Table 5: Effect of naringin, hesperidin, and their combinations on oxidative stress markers in different studied groups.
|
Groups Parameters |
Control |
CD |
CD + Nar |
CD + Hes |
CD + Nar + Hes |
|
OxLDL(pg/ml) |
6.06±0.67 |
9.02±0.87a |
6.13±0.78b |
7.65±0.64 |
7.15±1.31 |
|
SOD (U/ml) |
709.18±7.90 |
645.51±1.64a |
695.86±12.42b |
688.37±12.85b |
693.79±13.18b |
|
MDA(nmol/ml) |
3.29±0.04 |
5.12±0.35a |
4.26±0.12a,b |
4.50±0.43a,b |
4.24±0.005a,b |
Data are expressed as mean+/- standard error; percentage change versus control. a: significance versus control; b: significance versus CD; c: significance versus CD + Nar; d: significance versus CD + Hes.
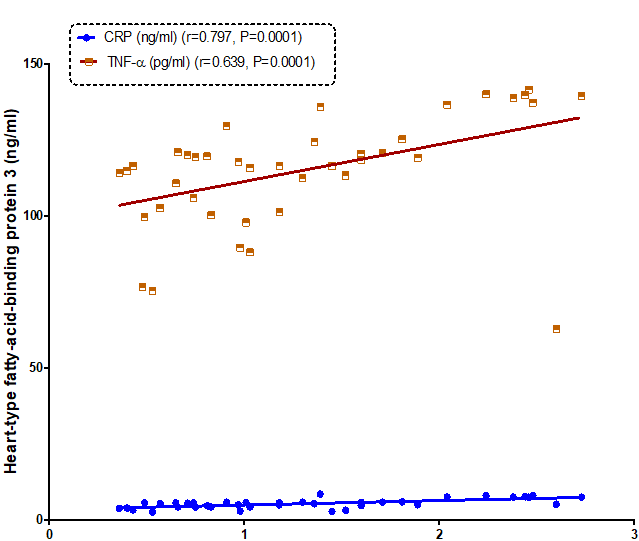
Figure 1: Correlation between heart-type fatty acid-binding protein 3 and C-reactive protein (CRP) and tumor necrosis factor –α (TNF-α) in all the studied groups.
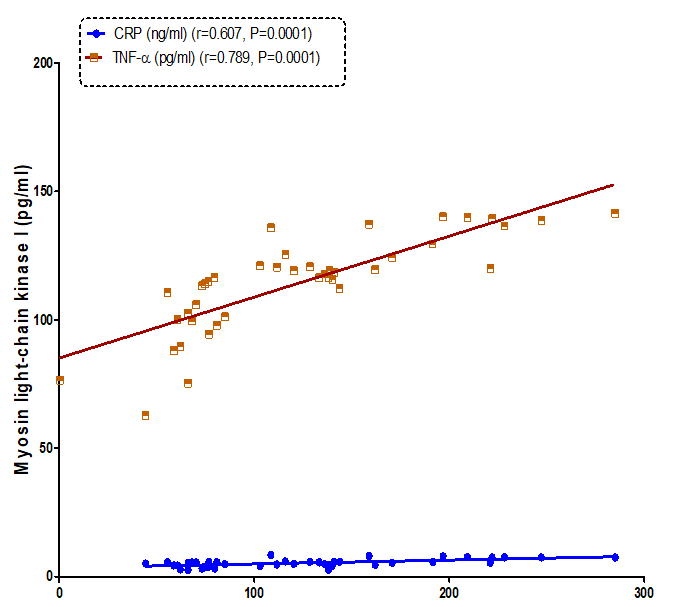
Figure 2: Correlation between Myosin light chain kinase I (pg/ml) and C-reactive protein (CRP) and tumor necrosis factor –α (TNF-α) in all the studied group.
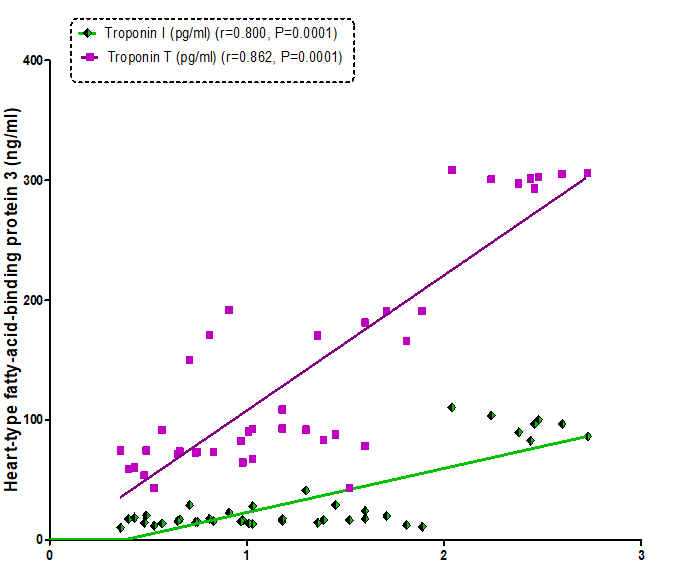
Figure 3: Correlation between heart-type fatty acid-binding protein 3 and Troponin I and Troponin T in all the studied groups.
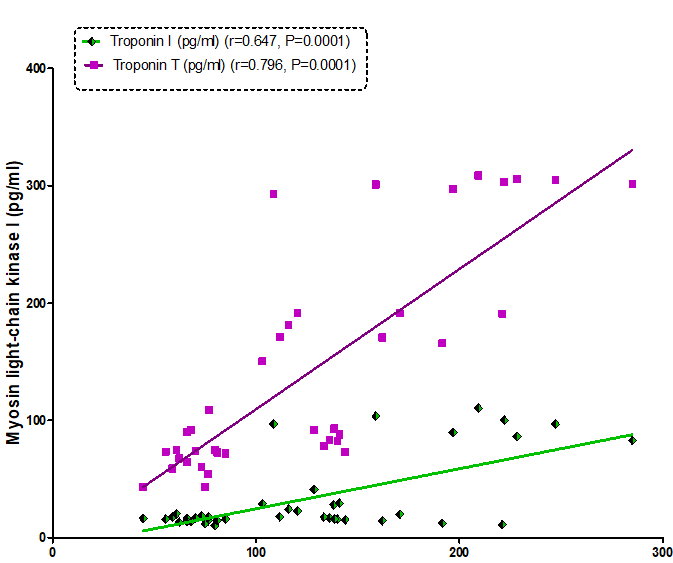
Figure 4: Correlation between myosin light chain kinase and Troponin I and Troponin T in all the studied groups.
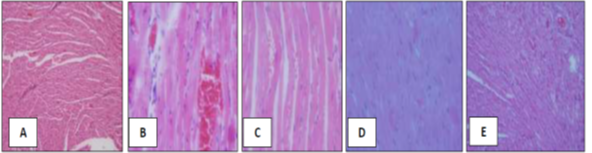
Figure 5: (A) photomicrograph of a healthy control group showing a normal histological structure of the heart in a healthy control group. (X60). The figures showed myocardial cells with prominent nuclei and blood vessels. Spiral shaped fibroblasts of connective tissues surrounding the myocardial smooth muscles. (B)Photomicrograph of heart section of Rats with induced cardiac damage (CD). (X60). The figures showed a loss of myocardial fibers and vacuolated cells with profuse bleeding. (C) Photomicrograph of heart section of Rats with induced cardiac damage (CD) treated with Naringin. (X60). The figures showed myocardial fibers and vacuolated cells with dilated blood vessels with less bleeding. (D) Photomicrograph of heart section of Rats with induced cardiac damage (CD) treated with Hesperidin. (X60). The figures showed myocardial cells with prominent nuclei and blood vessels. (E) Photomicrograph of heart section of Rats with induced cardiac damage (CD) treated with Naringin & Hesperidin. (X60). The figures showed myocardial cells with prominent nuclei and profuse blood.
In the last three figures, found the Spiral shaped fibroblasts of connective tissues surrounding the myocardial smooth muscles are also clearly seen.
DISCUSSION
This study demonstrated the propensity of Adriamycin to induce cardiac damage. The reported high levels of FABP supported this result, MYLK, cTn-I, and cTn-T as compared to the control group. These findings were similar to those reported Zhang et al. [23]. The study illustrated that Adriamycin induces oxidative stress, inflammation, and apoptosis. Furthermore, Setsuta et al.also found high levels of FABP, which was linked to the manifestation of cardiac diseases among patients suffering from chronic heart failure [24]. The rats that were treated with Adriamycin had lower levels of mRNA for myosin light chain in cardiac muscles. These alterations in the expression of genes in cardiocyte cultures and cardiac muscles explain the changes in the structure of the heart muscles and are linked to myofibrillar loss that is evident in adriamycin cardiac injury [25]. A previous study has provided evidence that Adriamycin impacts the expression of myocardial structure and regulatory proteins, such as MYLK [26].
Other biomarkers that are affected were the cardiac troponins troponin I/T (cTnI/T). These biomarkers are highly specific to cardiac dysregulation [27]. In the current study, there was an elevated level of cTn-I and cTn-T in the cardia dysfunction induced group. These findings were agreed with findings reported by Bertinchant et al., where a strong association between maximal level of serum cTnT, the extent of myocardial morphological changes, and echocardiographic LV diameters in the rat model [28]. In previous studies, it was reported that levels of LDH, CK-MB, and AST were high in rats after the administration of Adriamycin. The elevation of the LDH, CK-MB, and AST was a sign of damage to the heart muscles. The results corresponded with those previously reported by Nimbal and Koti [29]. Nimbal and colleagues also reported high levels of LDH and CK-MB in blood signifying damage in the heart muscles [30].
There are high levels of AST in heart tissues, and the presence of this enzyme in serum is an indication of damage in heart muscles and consequent release of the enzyme in blood [30]. The increase in heart lipid peroxides following treatment with adriamycin can explain the scenario [31].
The heart muscles secrete natriuretic peptides due to distension in the ventricular wall [32]. The findings of the current study demonstrated that there was a high level of BNP in the cardiac dysfunction induced group of the experimental mice relative to the control group. Similar findings were reported by ElGhandour et al.[33]. Our data also reported high levels of TNF-α in the CD group as compared to the control group. Abdel-Daim et al. reported similar results, where they reported that adriamycin-induced acute inflammation [34].
There was, however a significant increase in levels of ox-LDL and MDA. The levels of SOD were decreased in the CD group as compared to the control group. These findings could be explained by the fact that adriamycin can induce oxygen-derived free radical formation [35].
A previous study showed that naringin could potentially reduce cardiac dysfunction [36]. Moreover, it has been shown that Naringin has an anti-inflammatory, anti-oxidant and anti-apoptotic effect and can reduce the effects of myocardial injury in the experiment [37].
The results of the current study also showed a significant reduction in FABP, MYLK, cTn-I, and cTn-T levels in a group of experimental mice treated with naringin. These results were in agreement with those reported by Kanno et al. [38]. A study by Rajadurai and Prince demonstrated the positive effect of naringin in influencing the levels of cTnT and the activities of cardiac marker enzymes [39]. The levels of CK-MB, LDH, and AST in mice group treated with naringin significantly decrease confirming the results previously reported by Ahmed et al. [40]. Ahmed reported an increase in the activity of plasma CK-MB observed.
Besides, a major reduction in BNP was reported in a group of experimental rats treated with naringin. One of the explanations to this result that naringin can protect the heart muscles by controlling heart enzymes [41]. The result also showed a decrease in TNF-α and CRP levels in the mice CD group. Chen and colleagues have also reported that naringin reduces cardiovascular diseases [42, 43]. Other studies have shown that naringin has the propensity to regulate the release of SOD and MDA [36]. Natural compounds in citrus fruits can protect the body from oxidative stress by antioxidant activity and through the scavenging of free radicals [44].
The current study showed that there was a significant reduction in FABP, MYLK, cTn-I, and cTn-T levels in the hesperidin treated group. These findings are supported by the fact that Hesperidin has anti-carcinogenic, vascular protective, and lipid-lowering activities [45]. Our results were also in agreement with those reported by Li et al. [46]. Another intriguing finding was the activity of Hesperidin to reduce the function of plasma cardiac troponin T and I levels. This effect is an indication of Hesperidin in protecting the heart [47]. This activity can be linked to Hesperidin calcium channel blocking activity [48]. The current study has confirmed the reduction of cardiac markers including CK-MB, LDH and AST in a group administered with hesperidin. This finding can be explained by the fact that treatment with hesperetin led to reduction of adriamycin-induced DNA damage [21]. The reported result was supported by those previously obtained by Agrawal et al. [49]. Besides, we reported a major reduction in TNF-α and CRP in rat group administered with hesperidin and the result agreed with Rizza et al. [50]. Rizza reported positive effects of hesperidin on endothelial function.The current study result reported that there was a decrease in oxidative stress biomarkers, including ox-LDL and MDA, whereas SOD levels in the hesperidin administered the group. These findings corroborated with the findings reported by Jagdish et al. [51].
It has previously been established that hesperidin has a function in reducing oxidative stress. Emerging evidence shows that hesperidin protects cardiac tissue through its antihypertensive and antioxidant abilities [52]. The results of this study showed that there was a major reduction in oxidative stress markers, including ox-LDL and MDA. This result was in line with the findings reported by Jagdish et al. [51].
The data reported by the current study were confirmed by performing histopathological investigations on cardiac tissues. The investigations revealed that adriamycin intoxication caused serious myocardial degeneration manifested as myofibrillar loss, vacuolization, inflammation, and interstitial edema. The same pattern of histopathological alterations was previously reported in acute adriamycin-induced cardiotoxicity [53]. However, Naringin treatment did not cause observable changes in the structure of the heart muscles. This finding implied that naringin helped to reduce adriamycin-induced acute cardiotoxicity [54].
Microscopic examination of the heart tissues also suggested that adriamycin treatment caused morphological changes, including the reorganization of cellular arrangement and vacuolization. Treatment of rats with Hesperetin concurrently resulted in a reduction in alteration of cellular structure in the cardiac muscles [21].
A combination of treatment groups showed a significantly ameliorative effect in myocardial infarction as compared to the administration of single compounds such as in adriamycin-induced rats, with the reduced cTn-I, FABP3 and MYLK levels in serum analysis. However, the effects of cTn-T levels in combinatory administration were the same relative to the naringin group. The markers for cardiac damage showed a greater effect in combination administration group in the levels of CK-MB and AST. However, the LDH level in the naringin administered group showed a better amelioration compared to the combination-treated group.
CONCLUSION
The study findings demonstrated the powerful biological effects of naringin and hesperidin in the treatment of heart diseases. Both the biochemical and microscopic examinations revealed that naringin and hesperidin administration could protect the heart from Adriamycin-induced myocardial infarction. These compounds act through their antioxidant and anti-inflammatory effects.
REFERENCES
- Kiage-Mokua BN, de Vrese M, Kraus-Stojanowic I, Nielsen A, Roos N, Ghadimi D, Schrezenmeir J. Hypertriglyceridemia and CVD Risk Factors’ Reduction Using Lapacho Tea. International Journal of Pharmaceutical and Phytopharmacological Research (eIJPPR). 2018 Jun 1;8(3):39-45.
- KUMOSANI, T. A., ALAMA, M. N. & IYER, A. Cardiovascular diseases in Saudi Arabia. Prime Res Med, 2011 ; 1, 1-6.
- Areshidze DA, Mischenko DV, Makartseva LA, Kucher SA, Kozlova MA, Timchenko LD, Rzhepakovsky IV, Nagdalian AA, Pushkin SV. Some Functional Measures of the Organism of Rats at Modeling of Ischemic Heart Disease in Two Different Ways. Entomology and Applied Science Letters. 2018 Jan 1;5(4):19-29.
- Levenson, J. W., Skerrett, P. J. & Gaziano, J. M. Reducing the global burden of cardiovascular disease: the role of risk factors. Preventive cardiology, 2002 ; 5, 188-199.
- Faramrza Zadeh R, Shahabi Rabori V. Relationship between TIMI myocardial perfusion grade and ST resolution in evaluating the coronary reperfusion. Journal of Advanced Pharmacy Education and Research. 2018;8(1):23-31.
- Joyner, M. J. & Green, D. J. Exercise protects the cardiovascular system: effects beyond traditional risk factors. The Journal of physiology, 2009 ; 587, 5551-5558.
- Ahmed, A. M., Hersi, A., Mashhoud, W., Arafah, M. R., Abreu, P. C., Al Rowaily, M. A. & Al-Mallah, M. H. Cardiovascular risk factors burden in Saudi Arabia: The Africa Middle East Cardiovascular Epidemiological (ACE) study. J Saudi Heart Assoc, 2017 ; 29, 235-243.
- Danaei, G., Finucane, M. M., Lu, Y., Singh, G. M., Cowan, M. J., Paciorek, C. J., Lin, J. K., Farzadfar, F., Khang, Y.-H. & Stevens, G. A. 2011. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since : systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2· 7 million participants. The Lancet, 1980 ; 378, 31-40.
- Bai, J., Ma, M., Cai, M., Xu, F., Chen, J., Wang, G., Shuai, X. & Tao, K. Inhibition enhancer of zeste homologue 2 promotes senescence and apoptosis induced by doxorubicin in p53 mutant gastric cancer cells. Cell proliferation, 2014 ; 47, 211-218.
- Scully, R. E. & Lipshultz, S. E. Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovascular toxicology, 2007 ; 7, 122-128.
- Alkreathy, H., Damanhouri, Z. A., Ahmed, N., Slevin, M., Ali, S. S. & Osman, A.-M. M. Aged garlic extract protects against doxorubicin-induced cardiotoxicity in rats. Food and chemical toxicology, 2010 ; 48, 951-956.
- Šimůnek, T., Štěrba, M., Popelová, O., Adamcová, M., Hrdina, R. & Geršl, V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacological reports, 2009 ; 61, 154-171.
- Spallarossa, P., Garibaldi, S., Altieri, P., Fabbi, P., Manca, V., Nasti, S., Rossettin, P., Ghigliotti, G., Ballestrero, A. & Patrone, F. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. Journal of molecular and cellular cardiology, 2004 ; 37, 837-846.
- Assini, J. M., Mulvihill, E. E. & Huff, M. W. Citrus flavonoids and lipid metabolism. Current opinion in lipidology, 2013 ; 24, 34-40.
- Tripoli, E., La Guardia, M., Giammanco, S., Di Majo, D. & Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food chemistry, 2007 ; 104, 466-479.
- Sharma, A. K., Bharti, S., Ojha, S., Bhatia, J., Kumar, N., Ray, R., Kumari, S. & Arya, D. S. Up-regulation of PPARγ, heat shock protein-27 and-72 by naringin attenuates insulin resistance, β-cell dysfunction, hepatic steatosis and kidney damage in a rat model of type 2 diabetes. British Journal of Nutrition, 2011 ; 106, 1713-1723.
- Hajialyani, M., Hosein Farzaei, M., Echeverría, J., Nabavi, S. M., Uriarte, E. & Sobarzo-Sánchez, E. Hesperidin as a neuroprotective agent: A review of animal and clinical evidence. Molecules, 2019 ; 24, 648.
- Roohbakhsh, A., Parhiz, H., Soltani, F., Rezaee, R. & Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life sciences, 2015 ; 124, 64-74.
- Itoh, M., Iwai, K., Kotone-Miyahara, Y., Yamada, H., Ohno, H., Yamamoto, K., Tashima, M., Inoko, M., Nohara, R. & Uchiyama, T. Successful allogeneic bone marrow transplantation for acute myelogenous leukemia after drug-induced cardiomyopathy. The Tohoku journal of experimental medicine, 2004 ; 204, 85-91.
- Rajadurai, M. & Stanely Mainzen Prince, P. Preventive effect of naringin on cardiac markers, electrocardiographic patterns and lysosomal hydrolases in normal and isoproterenol-induced myocardial infarction in Wistar rats. Toxicology, 2007a ; 230, 178-188.
- Trivedi, P., Kushwaha, S., Tripathi, D. & Jena, G. Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Cardiovascular toxicology, 2011 ; 11, 215-225.
- Bancroft, N., Seip, H. & Sprengel, A. Implementing SAP R/3, 2nd edition'. Greenwich: Manning Pubilcations Co, 1998.
- Zhang, J., Clark, J. R., Herman, E. H. & Ferrans, V. J. Doxorubicin-induced apoptosis in spontaneously hypertensive rats: differential effects in heart, kidney and intestine, and inhibition by ICRF-187. Journal of molecular and cellular cardiology, 1996 ; 28, 1931-1943.
- Setsuta, K., Seino, Y., Ogawa, T., Ohtsuka, T., Seimiya, K. & Takano, T. Ongoing myocardial damage in chronic heart failure is related to activated tumor necrosis factor and Fas/Fas ligand system. Circulation Journal, 2004 ; 68, 747-750.
- Strauss, M., Rada, A., Tejero, F., Eacute, Lix, Hermoso, T. & Aacute. Heat Stress in Rat Adriamycin Cardiomyopathy: Heat Shock Protein 25 and Myosin Accumulation. Journal of Toxicologic Pathology, 2010 ; 23, 235-243.
- Ito, T., Muraoka, S., Takahashi, K., Fujio, Y., Schaffer, S. W. & Azuma, J. Beneficial effect of taurine treatment against doxorubicin-induced cardiotoxicity in mice. Taurine 7. Springer, 2009.
- Atas, E., Kismet, E., Kesik, V., Karaoglu, B., Aydemir, G., Korkmazer, N., Demirkaya, E., Karslioglu, Y., Yurttutan, N. & Unay, B. Cardiac troponin-I, brain natriuretic peptide and endothelin-1 levels in a rat model of doxorubicin-induced cardiac injury. Journal of cancer research and therapeutics, 2015 ; 11, 882.
- Bertinchant, J., Polge, A., Juan, J., Oliva-Lauraire, M., Giuliani, I., Marty-Double, C., Burdy, J., Fabbro-Peray, P., Laprade, M. & Bali, J. Evaluation of cardiac troponin I and T levels as markers of myocardial damage in doxorubicin-induced cardiomyopathy rats, and their relationship with echocardiographic and histological findings. Clinica Chimica Acta, 2003 ; 329, 39-51.
- Nimbal, S. & Koti, B. Cardioprotective Effect of Rosa centifolia against Doxorubicin-induced Myocardial Toxicity in Albino Rats. RGUHS J Pharm Sci, 2016 ; 6, 12-19.
- Nimbal, S. & Koti, B. Article Details Effect of Ethanolic Extract Fractions of Rosa centifolia in Doxorubicin-induced Myocardial Toxicity in Albino Rats, 2018.
- Boghdady, N. A. E. Antioxidant and antiapoptotic effects of proanthocyanidin and ginkgo biloba extract against doxorubicin‐induced cardiac injury in rats. Cell biochemistry and function, 2013 ; 31, 344-351.
- Iwanaga, Y., Nishi, I., Furuichi, S., Noguchi, T., Sase, K., Kihara, Y., Goto, Y. & Nonogi, H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. Journal of the American College of Cardiology, 2006 ; 47, 742-748.
- Elghandour, A. H., El Sorady, M., Azab, S. & Elrahman, M. Human heart-type fatty acid-binding protein as an early diagnostic marker of doxorubicin cardiac toxicity. Hematology Reviews, 2009 ; 1.
- Abdel-Daim, M. M., Khalifa, H. A. & Ahmed, A. A. Allicin ameliorates doxorubicin-induced cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Cancer chemotherapy and pharmacology, 2017 ; 80, 745-753.
- Mobaraki, M., Faraji, A., Zare, M., Dolati, P., Ataei, M. & Manshadi, H. D. Molecular mechanisms of cardiotoxicity: a review on major side-effect of doxorubicin. Indian Journal of Pharmaceutical Sciences, 2017 ; 79, 335-344.
- Xianchu, L., Lan, Z., Qiufang, L., Yi, L., Xiangcheng, R., Wenqi, H. & Yang, D. Naringin protects against lipopolysaccharide-induced cardiac injury in mice. Environmental toxicology and pharmacology, 2016 ; 48, 1-6.
- Adebiyi, O. A., Adebiyi, O. O. & Owira, P. M. Naringin reduces hyperglycemia-induced cardiac fibrosis by relieving oxidative stress. PLoS One, 2016 ; 11, e0149890.
- Kanno, S.-I., Shouji, A., Asou, K. & Ishikawa, M. Effects of naringin on hydrogen peroxide-induced cytotoxicity and apoptosis in P388 cells. Journal of pharmacological sciences, 2003 ; 92, 166-170.
- Rajadurai, M. & Stanely Mainzen Prince, P. Preventive effect of naringin on cardiac mitochondrial enzymes during isoproterenol‐induced myocardial infarction in rats: A transmission electron microscopic study. Journal of biochemical and molecular toxicology, 2007b ; 21, 354-361.
- Ahmed, K. M., Rana, A. & Dixit, V. Effect of Calotropis procera latex on isoproterenol induced myocardial infarction in albino rats. Phytomedicine, 2004 ; 11, 327-330.
- You, Q. & Wu, K. The cardiovascular pharmacology of Naringin j. Guangdong Medical, 2010 ; 31, 22.
- Chen, R., Sun, G., Wang, J., Zhang, H. & Sun, X. Naringin protects against anoxia/reoxygenation-induced apoptosis in H9c2 cells via the Nrf2 signaling pathway. Food & function, 2015 ; 6, 1331-1344.
- Habauzit, V., Sacco, S. M., Gil-Izquierdo, A., Trzeciakiewicz, A., Morand, C., Barron, D., Pinaud, S., Offord, E. & Horcajada, M.-N. Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone, 2011 ; 49, 1108-1116.
- Johnson, M. K. & Loo, G. Effects of epigallocatechin gallate and quercetin on oxidative damage to cellular DNA. Mutation Research/DNA Repair, 2000; 459, 211-218.
- Wang, X., Hasegawa, J., Kitamura, Y., Wang, Z., Matsuda, A., Shinoda, W., Miura, N. & Kimura, K. Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. Journal of pharmacological sciences, 2011 ; 117, 129-138.
- Li, X., Hu, X., Wang, J., Xu, W., Yi, C., Ma, R. & Jiang, H. Inhibition of autophagy via activation of PI3K/Akt/mTOR pathway contributes to the protection of hesperidin against myocardial ischemia/reperfusion injury. International journal of molecular medicine, 2018 ; 42, 1917-1924.
- Selvaraj, P. & Pugalendi, K. V. Hesperidin, a flavanone glycoside, on lipid peroxidation and antioxidant status in experimental myocardial ischemic rats. Redox Report, 2010 ; 15, 217-223.
- Morita O, Sasaki H & S, S. Calcium antagonists containing phenols. Pattent Japan Kokai Tokkyo Koho, 1992 ; 04, 822.
- Agrawal, Y. O., Sharma, P. K., Shrivastava, B., Ojha, S., Upadhya, H. M., Arya, D. S. & Goyal, S. N. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS One, 2014 ; 9, e111212.
- Rizza, S., Muniyappa, R., Iantorno, M., Kim, J.-A., Chen, H., Pullikotil, P., Senese, N., Tesauro, M., Lauro, D. & Cardillo, C. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. The Journal of Clinical Endocrinology & Metabolism, 2011 ; 96, E782-E792.
- Jagdish, K., Mehul, S. & Nehal, S. Effect of hesperidin on serum glucose, HbA1c and oxidative stress in myocardial tissue in experimentally induced myocardial infarction in diabetic rats. Pharmacognosy Journal, 2010 ; 2, 185-189.
- Wilmsen, P. K., Spada, D. S. & Salvador, M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. Journal of agricultural and food chemistry, 2005 ; 53, 4757-4761.
- Mantawy, E. M., El-Bakly, W. M., Esmat, A., Badr, A. M. & El-Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. European journal of pharmacology, 2014 ; 728, 107-118.
- Kwatra, M., Kumar, V., Jangra, A., Mishra, M., Ahmed, S., Ghosh, P., Vohora, D. & Khanam, R. Ameliorative effect of naringin against doxorubicin-induced acute cardiac toxicity in rats. Pharmaceutical biology, 2016 ; 54, 637-647.
