Changes in Phenolic Content and Antioxidant Activity between Twinings English breakfast Tea Bags and Opened Tea Bags
Ulfat M. Omar1, Hadeil M. Alsufiani1,2, Gadah A. Alshahrany1, Kholoud A. Alyami1, Laila M. Alzahrani1, Rasha A. Mansouri1*
1 Department of Biochemistry, Faculty of Science, King AbdulazizUniversity, Jeddah, KSA.
2 Experimental Biochemistry Unit, King FahadMedicalResearch Center, King AbdulazizUniversity, Jeddah, Saudi Arabia.
*Email: [email protected]
ABSTRACT
Tea is one of the most widely consumed beverages in the world and is recognized as having numerous beneficial health effects due to its phenolic content. The current study aimed to evaluate and compare the phenolic and flavonoid content of two forms of Twining English Breakfast Tea: tea bags (TBs) and opened tea bags (OTBs). The antioxidant activity was also assessed using DPPH, FRAP, and CUPRAC assays. The results obtained revealed that the OTB infusion exhibited significantly higher phenolic and flavonoid content than the TB infusion at 5 and 10 minutes. The OTB infusion also significantly showed higher iron and cupper reducing powers than the TB infusion at the same time points. However, both samples demonstrated the same scavenging activity across all infusion times. These results suggest that the tea bags used with Twinings English Breakfast Tea may affect the phenolic and flavonoid content released by the tea and subsequently influence the antioxidant ability of the tea as a reducing agent.
Key words: Polyphenol, Flavonoid, Antioxidants, Black tea, DPPH, FRAP, CUPRAC.
INTRODUCTION
Globally, tea is the most popular beverage after water [1, 2]. Indeed, tea consumption increased from 234 billion liters in 2013 to 289 billion liters in 2020 [3]. This volume is expected to grow further, with estimates that it will reach 297 billion liters by 2021 This fragrant beverage mainly consists of phenolic substances, flavonoids, caffeine, carbohydrates, organic acids, minerals, and volatile compounds [4].
Many scientific researchers have documented that the phenolic and flavonoid content present in tea are responsible for its antioxidant activity [5-9]. It is further well known that there is a direct correlation between the antioxidant activity of tea and health, with many studies showing that consuming tea offers multiple different health benefits, including anticancer, anti-inflammatory, anti-aging, and antibacterial advantages [10-14].
Researchers have classified tea into different types based on the processing method used: unfermented (green tea), partially fermented (white, yellow, and oolong tea), and completely fermented (black tea) [15, 16]. The global consumption of tea type varies, with black tea being the most popular and favored in Western countries [17]. Black tea is characterized by its copper-red infusion, high caffeine content, and strong aroma. However, many factors affect the quality and antioxidant properties of tea, such as the type of soil, leafage, manufacturing process, preparation method, and infusion time [18-22]. Moreover, many studies have demonstrated that particle size can modulate the antioxidant activity of tea and its polyphenol content [5, 23, 24].
The growing interest in the health benefits of tea and the fact that tea is a very popular beverage has led to many studies that have sought to determine the relationship between the biological properties exhibited by tea and the components of tea. Also, the trade market offers different forms of tea, either loose tea leaves or bagged tea, to satisfy consumers’ daily tea needs. However, to date, there has only been one study, which was conducted by our research group in 2019, that aimed to study the effects of the teabag on the antioxidant activity of tea. Mansouri et al. [25] conducted the study using three forms of Al-Kbous black tea, namely, tea bags (TBs), loose tea (LT), and opened tea bags (OTBs). In this study, we, therefore, aimed to investigate another well-known tea brand to highlight the best way of drinking black tea to achieve maximum biological benefits. Two forms of Twinings English Breakfast Tea were used in the study: TBs and OTBs. Scavenging 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, ferric reducing antioxidant power (FRAP), and cupric ion reducing antioxidant capacity (CUPRAC) assays were used to evaluate the antioxidant properties exhibited by each form of tea.
MATERIALS AND EXPERIMENTAL METHODS
Chemicals
Standard gallic acid, catechin, ferrous sulfate, and Trolox were purchased from Fisher Scientific (Loughborough, UK) for use in this study, while sodium carbonate (Na2CO3), Folin–Ciocalteu reagent, sodium nitrate (NaNO2), aluminum chloride (AlCl3), sodium hydroxide (NaOH), an acetate buffer, tripyridyl triazine (TPTZ), iron (III) chloride hexahydrate (FeCl3.6H2O), copper sulfate (CuSO4), neocuproine, ethanol, and DPPH were obtained from Sigma-Aldrich Chemical Company Ltd. (Poole, UK).
Tea Samples
Twinings English Breakfast Tea was purchased in tea bags from a local supermarket in Saudi Arabia and stored in a dry place at room temperature. Two different tea forms were examined in the current study: TBs and OTBs.
Preparation of the Tea Infusions
Two different beakers containing 200 ml of boiled water were prepared. One tea bag was infused in one beaker, while a new tea bag was opened and infused in the other beaker. Both samples were incubated for different steeping times (5, 10, and 15 minutes) at room temperature (24°C). The infusion times were selected because they reflect one of the traditional ways of drinking tea in Saudi Arabia in which the tea bag is left in the cup/teapot until the tea is consumed. The preparation of each tea infusion was carried out in triplicate.
Determination of the Total Phenolic Content
The total phenolic content (TPC) of the tea samples was determined using the Folin–Ciocalteu method [26]. Briefly, 0.5 ml of a tea infusion or standard solutions of 0.2, 0.4, 0.6, 0.8, and 1.0 mg/ml of gallic acid were placed in a volumetric flask that contained 2.4 ml of deionized water and 0.1 ml of a Folin–Ciocalteu reagent. After 5 minutes, 2 ml of 2% Na2CO3 was added, and the mixture was then incubated at room temperature. After 60 minutes of incubation, the absorbance of the samples was measured using a spectrophotometer at 750 nm.
Determination of the Total Flavonoid Content
The total flavonoid content was assessed using aluminum chloride colorimetric assay [22]. A 0.5 ml tea infusion or standard solutions of 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml of catechin were mixed with 2.2 ml of distilled water and 0.15 ml of 5% sodium nitrate (NaNO2). After 6 minutes, 0.15 ml of 10% AlCl3 was added to the mixture. After another 6 minutes of incubation, 2 ml of 4% NaOH was added, and the mixture was mixed well by a vortex. All the samples were incubated for 15 minutes, and the absorbance was subsequently measured using a spectrophotometer at 510 nm.
DPPH Radical Scavenging Assay
This assay was performed as described by Omar et al. [26] using synthetic free radical DPPH. A volume of 500 µl of tea infusion was mixed with 500 µl of 99.5% ethanol and 125 µl of 0.02% DPPH (prepared previously in ethanol). The mixture was then kept at room temperature in the dark for 60 minutes. The absorbance was subsequently measured with a spectrophotometer at 517 nm. The DPPH radical scavenging activity was expressed as an inhibition percentage and calculated using the following equation:
% inhibition = [(A of blank − A of the sample) / A of blank] ×100
Ferric Reducing Antioxidant Power Assay
A FRAP assay was prepared following the method proposed by Benzie and Strain [27] albeit with some modifications. The FRAP reagent was freshly prepared by mixing 0.3 mol/l of acetate buffer, TPTZ, and FeCl3.6H2O (20 mmol/l water solution) at a ratio of 10:1:1. To complete the method, 1.5 ml of freshly prepared FRAP reagent was mixed with 50 ul of tea infusion and then incubated for 30 minutes at 37°C. Following the incubation, the intensity of the blue color that had formed was measured against a blank using a spectrophotometer at 595 nm.
Cupric Ion Reducing Antioxidant Capacity Assay
The CUPRAC assay was carried out as described by Apaket al. [28] with a minor modification. A 0.1 ml tea infusion was mixed in a test tube with 0.3 ml of 5 mM CuSO4 and 0.3 ml of 3.75 mMneocuproine. The volume was then increased to 3.5 ml by adding distilled water. The obtained mixture was incubated for 30 minutes, and the absorbance was then measured with a spectrophotometer at 450 nm. The results were expressed in milligrams of Trolox per millimeter of extract.
Statistical Analysis
All the experimental methods were applied using three independent replications on three separate occasions. The results were analyzed statistically using GraphPad Prism software version 6.0. Statistical comparisons were performed using one-way analysis of variance (ANOVA) followed by the Mann–Whitney U test. All the data were presented as means ± SD, and differences at p < 0.05 were considered significant.
RESULTS
Total Phenolic Content
The TPC of the two tea infusions (TB and OTB) at different infusion times is presented in Figure 1. As demonstrated, the OTB exhibited significantly more phenolic content than the TB at 5 minutes (901.67 μg/ml vs 776.67 μg/ml, respectively; p< 0.001). A similar result was obtained at 10 minutes as the OTB showed significantly higher phenolic content (1034.67 μg/ml) than the TB (992.17 μg/ml; p < 0.01). At 15 minutes, there were no significant changes in either the infusion sample.
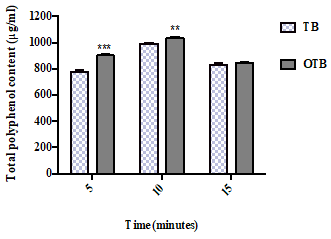
Figure 1. The total polyphenol content (μg/ml) of the teabag (TB) and the opened tea bag (OTB) at different infusion times. The data represent the mean of the three independent experiments ± SD. The statistical analysis was performed using a one-way variance analysis (ANOVA) followed by the Mann–Whitney U test. Significant differences between the two tea forms at the same time points are indicated by **p< 0.01 and ***p< 0.001.
Total Flavonoid Content
The TFC of the two tea infusions (TB and OTB) at different infusion times is presented in Figure 2. As shown in the figure, the flavonoid content continued to increase as the infusion time increased. The OTB exhibited significantly higher flavonoid content (230 μg/ml at 5 minutes and 286.67 μg/ml at 10 minutes) than the TB infusion sample, which measured 128.33 μg/ml at 5 minutes and 195 μg/ml at 10 minutes. However, at 15 minutes, neither sample showed any significant difference in flavonoid content.
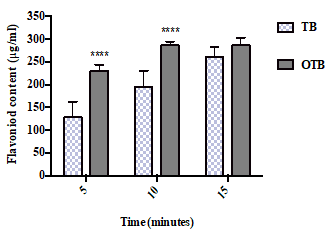
Figure 2. The total flavonoid content (μg/ml) of the teabag (TB) and the opened tea bag (OTB) at different infusion times. The data represent the mean of the three independent experiments ± SD. The statistical analysis was performed using a one-way variance analysis (ANOVA) followed by the Mann–Whitney U test. Significant differences between the tea forms at the same time points are indicated by ****p<0.0001.
DPPH Radical Scavenging Assay
Figure 3 demonstrates the DPPH radical scavenging activity exhibited by the TB and the OTB. There was no significant difference between either sample at any infusion time. Notably, the results obtained from this experiment indicated that the TB infusion was as powerful as the OTB infusion in scavenging DPPH radicals across all the infusion times.
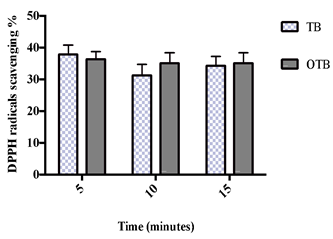
Figure 3. The DPPH radical scavenging activity of the teabag (TB) and the opened tea bag (OTB) at different infusion times. The data represent the mean of the three independent experiments ± SD. The statistical analysis was performed using a one-way variance analysis (ANOVA) followed by the Mann–Whitney U test.
Ferric Reducing Antioxidant Power Assay
The FRAP exhibited by the TB and OTB infusions is presented in Figure 4. A continuous increase in reducing power was observed for all the tea samples as the infusion time increased. The OTB infusion sample exhibited significantly higher reducing power (0.16 mMFe2+) than the TB infusion sample (0.13 mMFe2+) at 5 minutes. The same result was observed at 10 minutes when the OTB again exhibited a significantly higher antioxidant power (0.17 mMFe2+) than the TB sample (0.14 mMFe2+). However, there was no significant difference between the samples at 15 minutes.
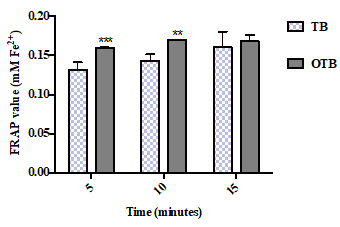
Figure 4. The FRAP of the tea bags (TB) and the opened tea bags (OTB) at different infusion times. The data represent the mean of three independent experiments ± SD. The statistical analysis was performed using a one-way variance analysis (ANOVA) followed by the Mann–Whitney U test. Significant differences between the tea forms at the same time points are indicated by **p < 0.01 and ***p < 0.001.
Cupric Ion Reducing Antioxidant Capacity Assay
The CUPRAC exhibited by the TB and OTB infusions is presented in Figure 5. The cupric ion reducing antioxidant power activity for both samples increased with the increasing infusion times; however, the OTB infusion sample exhibited a significantly higher reducing power (0.33 mML-1) than the TB infusion sample (0.27 mML-1) at 5 minutes. The same result was observed at 10 minutes when the OTB again exhibited a significantly higher antioxidant power (0.34 mML-1) than the TB sample (0.29 mML-1). Nevertheless, no significant difference was noted between the samples at the 15-minute time point.
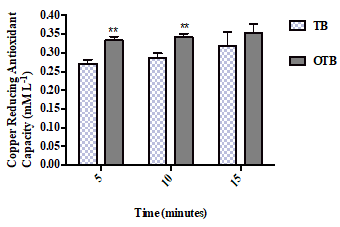
Figure 5. The CUPRAC of the teabag (TB) and the opened tea bag (OTB) at different infusion times. The data represent the mean of the three independent experiments ± SD. The statistical analysis was performed using a one-way variance analysis (ANOVA) followed by the Mann–Whitney U test. Significant differences between the tea forms at the same time points are indicated by **p < 0.01.
DISCUSSION
Tea is well known as an antioxidant beverage due to its phenolic content. Scientific researchers have revealed that the antioxidant properties exhibited by tea are influenced by a variety of factors, and manipulating these factors may enhance these properties. The trade market offers different forms of tea, either loose or bagged tea, to satisfy consumers’ daily tea needs. This study, therefore, aimed to analyze the effects of different forms of Twining English Breakfast Tea, namely, tea bags (TBs) and opened tea bags (OTBs), for their phenolic and flavonoid content and antioxidant activity at 5-, 10- and 15-minute infusion times.
It is common knowledge that the trade market uses finely ground tea leaves in their bagged tea to expose a larger surface area of tea particles to contact with hot water and thereby provide the fast release of phenolic and flavonoid compounds [7, 29]. In this study, since we depended on opening tea bags to create OTB samples, both forms were characterized by finely ground particles, and the only difference was the use of a teabag in the TB form. The study found that the OTB released significantly higher amounts of polyphenol and flavonoids at 5 and 10 minutes of infusion than the TB. These results may have been affected by the type of bag used in Twinings tea. The bags may have small pores or be made of a specific substance that decreases the contact between the tea particles and hot water and thus decreases the release of the phenolic and flavonoid content in the tea infusion [7, 29]. Using a TB requires a longer brewing time to allow sufficient release of the phenolic and flavonoid compounds. By way of comparison, our earlier study revealed that the OTB did not significantly affect the phenolic content when compared to the TB at 5, 10, and 15 minutes [25]. Also, many studies have shown that bagged tea has considerably richer phenolic and flavonoid content than loose tea since the latter has relatively larger particles and hence requires more time to allow the tea particles to open and release their phenolic content [20, 21, 29-31]. In contrast, the present study demonstrated that the type of bag used in Twinings tea had a greater impact on the phenolic and flavonoid content released during tea infusion when a comparison was done between the same particle size tea forms but one form included a tea bag and the other did not.
About the antioxidant activity of the two forms of tea, both the samples in our study had the same power to scavenge DPPH. These results confirmed our previous findings when our group examined the DPPH scavenging activity of different forms of Al-Kbous tea and found no significant difference between the TB and the OTB in terms of scavenging activity [25]. These results are also following those of Farooq and Sehgal [5] who found no noticeable differences between the radical scavenging effects of different forms of green tea (i.e., loose leaf, bagged, and matcha). On the other hand, Komes et al. [20] found that bagged tea exhibited the highest scavenging activity among powdered (matcha) and loose leaf tea. Makanjuola et al. [23] observed in their study that the extraction of different types of tea, including black tea, ginger, and a tea–ginger blend, with smaller tea particle sizes exhibited higher antioxidant properties [23]. The variations in the results between the studies may be explained by the presence of other variables that control the scavenging power exhibited by tea. Generally, the other studies comparing the results between bagged tea and loose tea attributed their findings to differences in the size of the tea particles, while in the current study, the only difference between our two samples was the presence of the teabag. This finding indicated that the tea bag used in Twinings tea has no impact on the scavenging activity exhibited by tea.
Although there was no significant difference in the scavenging DPPH activity between the two forms of tea used in this study, the OTB infusion exhibited significantly higher iron and cupric reducing power than the TB infusion. It was also noticeable that the activity in both tea infusions increased as the infusion time increased. The significant difference in reducing power between the two samples used in the current study may be explained by the higher phenolic content present in the OTB infusion than the TB infusion as it is well known that antioxidant properties have a significant correlation with phenolic content [18, 21, 22]. The study conducted by Nikniaz et al. (2016) on the loosely packed tea and bagged tea of five different tea brands revealed that the brewing time had a significant effect on the antioxidant activity shown by both forms of tea across all the samples. They further concluded that the bagged tea form of all the tea brands exhibited a higher ferric reducing power than the loosely packed tea, and this correlated with the amount of phenolic compound present in the infusion [30]. In their study, Komes et al. [20] found that bagged tea exhibited higher antioxidant activity than loosely packed tea. The results obtained by Komes et al. [20] and Nikniaz et al. [30] may be explained by the use of different parameters, including the concentration of the tea infusion, the infusion time, and the type of bag used in the bagged tea infusion, which affected the antioxidant activity. The findings of the current study regarding FRAP is in contrast with our previous study on Al-Kbous black tea [25] as the TBs exhibited significantly higher reducing power than the OTBs. Because the only changeable factor in the current study was the presence or absence of the bag used for Twinings tea, the results obtained indicated that the type of bag, including its pore size and the type of material used to manufacture it, is an important parameter that should be taken into consideration when evaluating the biological quality of tea.
CONCLUSIONS
This is the second novel study conducted by our group to examine the effects of tea bags in commercial use on tea quality. Two forms of Twinings English Breakfast Tea, namely, TBs and OTBs, were used in the current study. We found that the OTB allowed more phenolic and flavonoid content to be released in the tea infusion over a shorter period than the TB. Accordingly, a higher antioxidant power was exhibited by the OTB infusion when compared to the TB infusion. These findings demonstrate that the bag used to package tea has a high impact on the phenolic and flavonoid content and therefore the antioxidant ability of the tea. In future studies, more different tea trademarks (i.e., commercial brands) should be investigated to study the effects of the bags used on the quality of the tea. Also, studying the materials used as well as the pore size of the bags may explain the variations we noticed in our studies and reveal the best type of bags to use to preserve the quality of tea.
REFERENCES
- Aburawi SM, Owheda MA, Al-Jadid GA. Effect of Black Tea Preparation on Vitamin C Absorption in Albino Rat Ileum Using Everted Gut Sac Technique. Pharmacophores. 2019;10(6):9-13.
- Lakshmi T, Balusamy SR, Parameswari R. A review on green tea catechins in oral health management. J. Adv. Pharm. Educ. Res. 2017;7(3):178-89.
- Annualteaconsumptionworldwide 2013-2021. (n.d.). Retrieved July 22, 2019, from Statistawebsite: https://www.statista.com/statistics/940102/global-tea- consumption
- Harbowy, M. E., Balentine, D. A., Davies, A. P., Cai, Y. Tea Chemistry. critical reviews in Plant Sciences, 2010; 16 (5), 415-480. DOI:10.1080/07352689709701956
- Farooq, S., Sehgal, A. Antioxidant activity of different forms of green tea: loose-leaf, bagged, and matcha. Current Research in Nutrition and Food Science Journal, 2018; 6 (1), 35-40.
- Karori, S. M., Wachira, F. N., Wanyoko, J. K., Ngure, R. M. Antioxidant capacity of different types of tea products. African Journal of Biotechnology, 2007; 6 (19).
- Yang, J., Liu, R. H. The phenolic profiles and antioxidant activity in different types of tea. International Journal of Food Science &Technology, 2013; 48 (1), 163-171.
- Yashin, A., Yashin, Y., Nemzer, B. Determination of AntioxidantActivity in TeaExtracts, and Their Total Antioxidant Content. American Journal of Biomedical Sciences, 2011; 322-335. DOI:10.5099/aj110400322
- Khozaymeh F, Golestannejad Z, Shirzad M, Mojtahedi N. Growth Inhibition and Cytotoxicity Effect of Green Tea Extract on Squamous Cell Carcinoma Cell Line: An in Vitro Study. Ann. Dent. Spec. 2017;5(3):89-92.
- Chacko, S. M., Thambi, P. T., Kuttan, R., Nishigaki, I. Beneficial effects of green tea: a literature review. Chinese medicine, 2010; 5 (1), 13.
- Cooper, R., Morre`, D. J., Morre`, D. M. MedicinalBenefits of Green Tea: Part I. Review of Noncancerhealth benefits. The Journal of Alternative and complemementaryMedicine, 2005; 11(3), 521-528.
- Khan, N., Mukhtar, H. Teapolyphenols in the promotion of human health. Nutrients, 2019; 11 (1), 39.
- Khozaymeh, F., Golestannejad, Z., Shirzad, M., Mojtahedi, N. Growth Inhibition and CytotoxicityEffect of Green TeaExtract on SquamousCellCarcinomaCell Line: An in Vitro Study. Annals of Dental Specialty, 2017; 5 (3), 89-92.
- Lakshmi, T., Balusamy, S. R., Parameswari, R. A review on green tea catechins in oral health management. Journal of Advanced Pharmacy Education &Research, 2017; 7 (3).
- Koch, W., Kukula-Koch, W., Komsta, Ł3. Black TeaSamplesOrigin Discrimination UsingAnalytical Investigations of SecondaryMetabolites, AntiradicalScavengingActivity, and ChemometricApproach. Molecules, 2018; 26; 23 (3). pii: E513. DOI: 10.3390/molecules23030513.
- Yi, T., Zhu, L., Peng, W. L., He, X. C., Chen, H. L., Li, J., Yu, T., Liang, Z., Zhao, Z. Z., Chen, H. Comparison of ten major constituents in seven types of processed tea using HPLC-DAD-MS followed by principal component and hierarchical cluster analysis. Food Science and Technology, 2015; 62 (1). DOI: 10.1016/j.lwt.2015.01.003
- Ng TP., Aung KC., Feng, L., Feng, L., Nyunt MS, Yap KB. Tea consumption and physical function in older adults: a cross-sectional study. Journal of NutrHealthAging, 2014; 18 (2):161-166. DOI: 10.1007/s12603-013-0354-7.
- Al-Hafez, M., Kheder, F., AlJoubbeh, M. Polyphenols, flavonoids, and (-) epigallocatechin gallate in tea leaves and in their infusions under various conditions. Nutrition & Food Science, 2014; 44 (5), 455-463. DOI:10.1108/nfs-10-2013-0119
- Astill, C., Birch, M. R., Dacombe, C., Humphrey, P. G., Martin, P. T. Factors affecting the caffeine and polyphenol contents of black and green tea infusions.Journal of agricultural and food chemistry, 2001; 49 (11), 5340-5347.
- Komes, D., Horžić, D., Belščak, A., Ganić, K. K., Vulić, I. Green tea preparation and its influence on the content of bioactive compounds. Food research international, 2010; 43 (1), 167-176.
- Kyle, J. A., Morrice, P. C., McNeill, G., Duthie, G. G. Effects of infusion time and addition of milk on content and absorption of polyphenols from black tea. Journal of Agricultural and Food Chemistry, 2007; 55 (12), 4889-4894.
- Roshanak, S.; Rahimmalek, M.; Goli, S.A. Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. Journal of Food Science and Technology, 2015; 53:721-729
- Makanjuola, S. A. Influence of particle size and extraction solvent on antioxidant properties of extracts of tea, ginger, and tea–ginger blend. Food science & nutrition, 2017; 5 (6), 1179- 1185.
- Zaiter, A., Becker, L., Karam, M. C., Dicko, A. Effect of particle size on antioxidant activity and catechin content of green tea powders. Journal of food science and technology, 2016; 53 (4), 2025-2032.
- Mansouri, A. Rasha., Alsufiani, M. Hadeil., Alshahrany, A. Gadah., Alyami, A. Kholoud, Laila M. Alzahrani, M. Laila., Yamani, O. Raghad., Shorbaji, M. Ayat., Omar, M. Ulfat. Comparative Study of Total Polyphenol and AntioxidantActivity in different forms of Al-Kbous Black Tea. Journal of BiochemicalTechnology, 2019; 4, 11-15.
- Omar, U., Shorbaji, A., Arrait, E., Al-Agha, T., Al-Marzouki, H., Al Doghaither, H., Al-Ghafari, A. Comparative Study of the antioxidant activity of TwoPopular Green TeaBeveragesAvailable in the Local Market of Saudi Arabia. Natural Science, 20016; 8 (6), 227-234.
- Benzie, I. F., Strain, J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry, 1996; 239 (1), 70-76.
- Apak, R., Gu ̈c ̧lu ̈, K., O ̈zyu ̈rek, M., Karademir, SE., Altun, M. Total antioxidant capacity assay of human serum using copper(II)- neocuproine as chromogenicoxidant: the CUPRAC method. Free RadicRes, 2014; 39, 949–961
- Sharpe, E., Hua, F., Schuckers, S., Andreescu, S., Bradley, R. Effects of brewing conditions on the antioxidant capacity of twenty-four commercial green tea varieties. Food Chemistry, 2016; 192, 380-387.
- Nikniaz, Z., Mahdavi, R., Ghaemmaghami, S. J., Yagin, N. L., Nikniaz, L. Effect of different brewing times on antioxidant activity and polyphenol content of loosely packed and bagged black teas (Camellia sinensis L.). Avicenna journal of phytomedicine, 2016; 6 (3), 313.
- Rusak, G., Komes, D., Likić, S., Horžić, D., Kovač, M. Phenolic content and antioxidative capacity of green and white tea extracts depending on extraction conditions and the solvent used. Food Chemistry, 2008; 110 (4), 852-858.
