Efficacy and Safety of Sacubitril/valsartan in Patients with Heart Failure
Waleed Alhabeeb 1, Fakhr Al Ayoubi1, Ahmad Hayajneh1, Fayez Elshaer 1,2*
1College of Medicine, King Fahad Cardiac center, King Saud University, Riyadh, Saudi Arabia.
2National Heart Institute, Egypt.
* Email: felshaer @ ksu.edu.sa
ABSTRACT
Objective: Clinical trial data on sacubitril/valsartan are important. Considering that real-world data have important clinical implications, we aimed to report the efficacy and safety of sacubitril/valsartan in patients with heart failure (HF) in Saudi Arabia.Methods: This was a pilot prospective clinical trial consisting of adult patients (≥18 years old) with HF (left ventricular ejection fraction [EF] ≤40%) who were taking angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers for at least 1 month before presenting to the clinic and who were eligible for sacubitril/valsartan. Results: A total of 20 patients with a mean age of 60.75 years (range, 33–81 years) were included. At presentation, the majority of the patients presented with New York Heart Association (NYHA) class II symptoms (15/20), EF <30% (16/20), and ischemic etiology (15/20). After sacubitril/valsartan treatment initiation, most patients symptomatically improved to a lower NYHA class by 6 months, which was maintained until 12 months. N-terminal pro-B-type natriuretic peptide decreased from baseline at 6 and 12 months. During follow-up, no serious adverse events were reported. Complete echocardiogram data were available; however, no change in echocardiogram parameters was observed. Conclusions: In a real-world setting, sacubitril/valsartan improved HF symptoms, maintained acceptable resting heart rates, did not affect blood pressure, and did not lead to serious adverse events.
Key words: Heart failure; sacubitril/valsartan; ARNi; natriuretic peptides; real-world study.
INTRODUCTION
Heart failure is a complex syndrome defined by progressive neurohormonal activation and alteration in autonomic control [1]. Although angiotensin-converting enzyme (ACE) inhibitors have been the standard treatment of heart failure with reduced ejection fraction (HFrEF) for almost 25 years [2], combined inhibition of the renin-angiotensin system and neprilysin represents a novel pharmacological strategy with a potential to attenuate vasoconstriction and sodium retention and delay cardiac and vascular remodeling [1, 3]. Although the dual inhibition of the renin-angiotensin system and neprilysin showed superiority in experimental studies compared to either approach alone, clinical trials reported serious angioedema [2]. Hence, to minimize the risk of serious angioedema, a first-in-class angiotensin receptor neprilysin inhibitor-containing sacubitril (a neprilysin inhibitor) and valsartan (an angiotensin II receptor blocker [ARB]) called LCZ696 was designed [2].
In the pivotal phase III double-blind trial, in addition to the recommended therapy, PARADIGM-HF, (Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure) 8442 patients presenting with New York Heart Association (NYHA) class II-IV and twice daily, 200 mg of sacubitril/valsartan were to be received by randomized reduced ejection fraction of ≤40%. Compared to enalapril, sacubitril/valsartan resulted in a 20% reduction in the risk of death from cardiovascular causes, a 21% reduction of heart failure hospitalizations, a 16% reduction in the risk of all-cause mortality, and cardiovascular causes or first hospitalization for heart failure reduced by 20% (p<0.001 for all). Additionally, the trial was stopped prematurely, after a median follow-up of 27 months, due to the superior benefits of sacubitril/valsartan over enalapril [2]. Based on the obtained results, the US and European, with regulation, approved the sacubitril/valsartan. It also approved its guideline-based recommendations for treating selected patients with HFrEF [3].
This is the first study to report the real-word efficacy and safety of sacubitril/valsartan in selected patients with heart failure at King Fahad Cardiac Center, Riyadh, Saudi Arabia.
PATIENTS AND METHODS
Study Design
This was a pilot prospective clinical trial conducted at King Fahad Cardiac Center, King Saud University Medical City, Riyadh, Saudi Arabia. Reports reviewed were those of patients with heart failure presenting to the clinic after January 2016 and who were eligible for Entresto® (Sacubitril/valsartan; Novartis Pharmaceuticals Corporation). A heart failure cardiologist was responsible for patient selection, and a study nurse was responsible for data collection and entry. Based on protocols and ethical guidelines of the 1975 Declaration of Helsinki as shown before approval by the institution’s human research committee, the study was conducted.
Study subjects
Subjects aged ≥18 years with left ventricular ejection fraction ≤40% and NYHA class II-IV symptoms and who had been taking ACE inhibitors or ARBs for at least 1 month were included in the study. Subjects with symptomatic hypotension, systolic blood pressure less than 95 mmHg, estimated glomerular filtration rate less than 30 mL/min/1.73 m2, serum potassium over 5.3 mmol/L were excluded from the study. Also, pregnant and lactating women, and subjects with a history of angioedema were not part of the study.
Study procedures
The patient was required to attend a follow-up visit once every 2 weeks in the first month after starting sacubitril/valsartan and subsequently once a month thereafter for efficacy and safety monitoring for up to one year. According to the investigator’s clinical judgment, all study subjects were dosed. At baseline and each subsequent follow-up visit, the patient’s blood pressure and heart rate together with jugular venous pressure and serum potassium, serum sodium, serum creatinine, and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) levels were monitored.
In a subset of 15 patients, an echocardiogram was conducted to evaluate right ventricular dimension, end-diastolic and end-systolic dimensions, interventricular septum, posterior wall thickness, left atrium size, ejection fraction, fractional shortening, E velocity, A velocity, E/A ratio, E/e ratio, tricuspid annular plane systolic excursion, longitudinal strain (basal), mitral regurgitation grade, systolic pulmonary artery pressure, pericardial effusion, isovolumic relaxation time, deceleration time, tricuspid regurgitation severity, and left atrial volume index.
Safety evaluations were conducted at each follow-up visit and included evaluating laboratory results and asking patients the following five key questions:
- Do you experience shortness of breath or fatigue with minimal activity?
- Do you have swelling in your legs and/or abdomen?
- How many pillows do you use while sleeping?
- Do you restrict fluid and salt intake?
- Have you experienced any side effects (e.g., dry cough, itching)?
Study outcomes
The main outcome was the number of patients whose NYHA class symptoms changed from the baseline. The other outcomes evaluated were the change in NT-pro-BNP (pg/mL), heart rate (beats per minute), and systolic and diastolic blood pressure (mmHg). Blood pressure was classified according to the American College of Cardiology/American Heart Association: normal blood pressure (systolic reading <120 mmHg and diastolic reading <80 mmHg), increased blood pressure (systolic reading 120–129 mmHg and diastolic reading <80 mmHg), stage 1 hypertension (systolic reading 130–139 mmHg or diastolic reading 80–89 mmHg), and stage 2 hypertension (systolic reading ≥140 mmHg and diastolic reading ≥90 mmHg) [4]. The mean change in echocardiogram from baseline and after 6 months of treatment was recorded. The key safety outcomes were the mean serum creatinine (µmol/L), serum potassium (mmol/L), serum sodium (mmol/L), the rate of hospitalization, and serious adverse events.
Statistical analysis
Descriptive statistics were used to evaluate data collected at baseline and throughout treatment. Changes from baseline to 6 months and 12 months following treatment initiation were evaluated using the mean (± standard deviation, SD) change from baseline. Patients’ creatinine (50–110 µmol/L), potassium (3.5–5.1 mmol/L), and sodium (135–145 mmol/L) levels were calculated at baseline, 6 months, and 12 months.
RESULTS
Patient profile
In this ongoing chart review, data from 20 patients (13 males/7 females) were analyzed. The average age was 60.75 years (range, 33–81 years). Most patients had NYHA class II (15/20), while the remaining five patients had NYHA class III symptoms. Most patients (16/20) presented with ejection fraction ≤30%, of which three patients presented with severe heart failure (ejection fraction ≤15%). Fifteen of the 20 patients enrolled with HFrEF had ischemic etiology. History of comorbidities and medication use are shown in Table 1. Medical history showed that the majority of these patients (13/20) were taking lisinopril once daily with doses ranging from 2.5 mg to 20 mg. Other medications used before the study included candesartan, valsartan, and irbesartan.
Of the 20 patients evaluated, seven had a history of hospitalization. Two of the seven patients received an implantable cardioverter-defibrillator. Four of the seven patients were diagnosed with decompensated congestive heart failure, two of whom underwent percutaneous coronary intervention. Five of the seven patients visited the emergency room 2 or 3 times after hospital discharge, with three patients requiring intravenous furosemide in 6 months.
Efficacy
The majority of the patients on treatment with sacubitril/valsartan had a lower NYHA class by 6 months, which was maintained up to 12 months, compared to the baseline (Figure 1).
The mean decrease in NT-pro-BNP from baseline was -935.65 pg/mL (SD, 3436.84) at 6 months (mean [SD] NT-pro-BNP at baseline, 4656.17 pg/mL [3509.44]; mean [SD] NT-pro-BNP, 3253.90 pg/mL [3462.55]) and -1481.80 pg/mL (SD 3812.00) at 6 months; mean [SD] NT-pro-BNP at 12 months, 2708.75 pg/mL [2988.47]).
The mean resting heart rate was 74.90 beats per minute (±14.93) at baseline, 68.90 beats per minute (±12.38) at 6 months, and 69.70 beats per minute (±12.73) at 12 months (Figure 2).
The mean change in heart rate from baseline was -6.00 beats per minute (±15.76) at 6 months and -5.20 beats per minute (±17.64) at 12 months. The mean systolic blood pressure decreased by -9.95 mmHg (±20.39) at 6 months and -4.30 mmHg (±22.81) at 12 months. The mean diastolic blood pressure decreased by -5.85 (±11.01) at 6 months and -3.55 mmHg (±12.19) at 12 months. (Figure 3, 4, 5)
Echocardiogram findings
Of the 20 patients recruited in the study, complete echocardiogram data were recorded (Appendix 1). Based on the results obtained, 6 months’ treatment with sacubitril/valsartan improved the end-diastolic and end-systolic dysfunction, E/A and E/e ratio, systolic pulmonary artery pressure, left atrium thickness and left atrial volume index. Moreover, none of the patients presented with pericardial perfusion before or after starting sacubitril/valsartan treatment. The mean change in right ventricular dysfunction, interventricular septum thickness, posterior wall thickness, E and A velocity, and tricuspid regurgitation severity was clinically insignificant. The median NT-pro-BNP was 3697 pg/mL before treatment and 1952 pg/mL 6 months after sacubitril/valsartan treatment.
Safety
In general, sacubitril/valsartan was well tolerated, and the most common serious adverse effects, which were considered nonsignificant, were hypotension with dizziness, an increase in creatinine levels, hyperkalemia, and angioedema. Allergy (defined as dry cough and itching) and skin lesions were the most common non-serious adverse events reported by the patients. However, the mean serum creatinine level increased by 9 μmol/L at 6 and 12 months compared to the baseline level. Normal serum creatinine levels were observed in 40% of patients at baseline (n = 8), 30% of patients at 6 months (n = 6), and 40% of patients at 12 months (n = 8) (Table 2).
At 6 and 12 months, the mean serum potassium and sodium levels were similar to the baseline levels, with nonremarkable posttreatment increase; the majority of patients had normal serum potassium levels at baseline (n = 19, 95%), 6 months (n = 20, 100%), and 12 months (n = 18, 90%). Additionally, most patients had normal serum sodium levels at baseline (n = 16, 80%), 6 months (n = 16, 80%), and 12 months (n = 14, 70%). During follow-up, four patients required hospitalization after sacubitril/valsartan treatment initiation, of which one patient died in the hospital. Of the three living hospitalized patients, two were diagnosed with decompensated heart failure and one received an implantable cardioverter-defibrillator.
DISCUSSION
The prognosis of patients with heart failure remains poor, despite the availability of effective pharmacological and non-pharmacological interventions. The life expectancy of a patient being treated for heart failure is reduced, and the worsening of chronic heart failure often leads to frequent hospitalizations, which in turn impairs a patient’s quality of life [5].
In this observational study, sacubitril/valsartan resolved symptoms in all patients, indicated by lowering in NYHA class after 3 months of treatment compared to baseline. These effects appear to persist up to 6 months after treatment. Although NYHA classification is a functional/symptomatic score that does not take into account the underlying cardiac disorder that will almost inevitably progress [5], a decrease in NYHA class is considered a good prognostic factor in terms of survival and disease severity.
NT-pro-BNP is a cardiac biomarker that is highly significant in heart failure diagnosis and prognosis [6, 7]. A decrease in its value indicates an improvement in disease progression and patient prognosis [6, 7]. In this study, NT-pro-BNP decreased from baseline at each follow-up visit, with a large change observed as early as 2 weeks (-1346.23 pg/mL) and 1-month (-1609.23 pg/mL) post-treatment.
The mean heart rate appears to be consistent throughout the follow-up period, with 69 beats per minute at 6 months and 70 beats per minute at 12 months. Since the risk of cardiovascular mortality in patients with CHF increases consistently if the heart rate exceeds 80 bpm [8], our study showed that sacubitril/valsartan not only helps maintain a stable heart rate on treatment but also reduces the risk of cardiovascular complications or death associated with fluctuating heart rate.
Sacubitril/valsartan has been shown to lower blood pressure [9], is contraindicated in subjects with symptomatic hypotension or a systolic blood pressure <95 mmHg, and is used as a safety marker during treatment [10]. Although the mean blood pressure readings decreased at 6 and 12 months compared to the baseline, there were no recorded cases of symptomatic hypotension.
Echocardiogram data showed no statistically significant changes recorded in any of the 20 parameters evaluated before and after treatment. Although no significant changes in echocardiogram parameters were observed, no deterioration occurred and an overall trend toward improvement was observed. However, this observation is limited by the short follow-up and a small number of patients.
The main serious adverse events observed in this group of patients were hypotension, increased creatinine levels, and hyperkalemia. This was consistent with the results of the PARADIGM-HF trial wherein cases of symptomatic hypotension were reported in 14% of patients [2]. Although the percentage increase in serum creatinine was approximately 8% at months 6 and 12, this appeared to be a numerical increase that was not clinically relevant.
Although the efficacy of sacubitril/valsartan shown in clinical trials is compelling, its careful application in a real-world setting will have important implications for treatment selection, health service planning, and policymaking [11-13].
Study limitations
The key limitation of this study is that it included a relatively small number of patients. Although descriptive statistics were used, all recorded trends may have occurred by chance and may not be representative of the current situation. Results would need to be verified using larger observational studies. Despite the relatively short period of follow-up, sacubitril/valsartan was found to be effective in a real-life setting. Since the study is still underway, we anticipate that these trends will be replicated by the larger sample size in the long term.
CONCLUSIONS
In a real-world setting, sacubitril/valsartan improved HF symptoms, maintained acceptable resting heart rates, did not affect blood pressure, and did not lead to serious adverse events.
ACKNOWLEDGMENTS
Investigator support unit at KKUH
Funding
No specific grant was given to the researchers for carrying out this research.
Declaration of conflicting interests
No conflict of interest was declared by the authors.
REFERENCES
- Kaplinsky, E., Sacubitril/valsartan in heart failure: latest evidence and place in therapy. Ther Adv Chronic Dis, 2016. 7(6): 278-290.
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med, 2014; 371(11): 993-1004.
- Vaduganathan, M., Desai, A.S. Angiotensin-Neprilysin Inhibition as a Paradigm for All? Curr Cardiol Rep, 2016. 18(11): 115.
- Whelton PK, Carey RM, Aronow WS. Acc/aha/aapa/abc/acpm/ags/APhA/ASH/ASPC/nma/pcna guideline for the prevention, Detection, evaluation, and management of high blood pressure in adults: a Report of the American College of Cardiology/American heart Association. Task force on clinical practice guidelines//J. Am. Coll. Cardiol.-2017.-Nov 13. Почки. 2018;7(1).
- Mosterd, A., A.W. Hoes, Clinical epidemiology of heart failure. Heart, 2007. 93(9): 1137-46.
- Palazzuoli A, Gallotta M, Quatrini I, Nuti R. Natriuretic peptides (BNP and NT-proBNP): measurement and relevance in heart failure. Vasc Health Risk Manag, 2010. 6: 411-8.
- Iqbal N, Wentworth B, Choudhary R, Landa AD, Kipper B, Fard A, Maisel AS. Cardiac biomarkers: new tools for heart failure management. Cardiovasc Diagn Ther, 2012. 2(2): 147-64.
- Dobre D, Borer JS, Fox K, Swedberg K, Adams KF, Cleland JG, Cohen‐Solal A, Gheorghiade M, Gueyffier F, O'Connor CM, Fiuzat M. Heart rate: a prognostic factor and therapeutic target in chronic heart failure. The distinct roles of drugs with heart rate-lowering properties. Eur J Heart Fail, 2014. 16(1): 76-85.
- Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomized, double-blind, placebo-controlled, active comparator study. Lancet, 2010. 375(9722): 1255-66.
- Elmasry DM, Elnahas NG, Khorshid H, Rahmy AF. The effect of low-frequency neuromuscular stimulation on sympathetic activity in advanced heart failure. J. Adv. Pharm. Educ. Res. 2019; 9(4):29-35.
- Darkhor S, Estebsari F, Hosseini M, Charati JY, Vasli P. Effect of health promotion intervention on Nurses’ healthy lifestyle and health-promoting behaviors: RCT study. J. Adv. Pharm. Educ. Res. 2018; 8(1):109-114.
- Ren-Zhang L, Chee-Lan L, Hui-Yin Y. The awareness and perception on Antimicrobial Stewardship among healthcare professionals in a tertiary teaching hospital Malaysia. Arch. Pharma. Pract 2020; 11(2):50-9.
- Martinez Faller E, Therese Hernandez M, Mark Hernandez A, Risia San Gabriel J. Emerging Roles of Pharmacist in Global Health: An Exploratory Study on their Knowledge, Perception and Competency. Arch. Pharma. Pract. 2020; 11(1):40-6.
Table 1. Patient demographics.
|
Patient demographics |
N = 20 |
|
Sex [n/N] |
|
|
Male |
13/20 |
|
Female |
7/20 |
|
Age (years) (mean [range]) |
60.75 (33–81) |
|
Body mass index (kg/m2) (mean) |
28.39 |
|
Heart rate (beats/min) (mean [range]) |
74.9 (55–103) |
|
Systolic blood pressure (mmHg) (mean [range]) |
123.35 (96–154) |
|
Diastolic blood pressure (mmHg) (mean [range]) |
69.4 (50–90) |
|
Jugular venous pressure (cm) (mean [range]) |
14.3 (10–20) |
|
Clinical features of heart failure |
|
|
Ischemia (n/N) |
15/20 |
|
Left ventricular ejection fraction (%) |
26 (13–40) |
|
N-terminal pro-B-type natriuretic peptide (pg/mL) |
3162 (82–11,915) |
|
NYHA functional class (n/N) |
|
|
Class II |
15/20 |
|
Class III |
5/20 |
|
Serum creatinine (µmol/L) (mean [range]) |
120.15 (63–178) |
|
Serum potassium (mmol/L) (mean [range]) |
4.46 (3.0–5.1) |
|
Serum sodium (mmol/L) (mean [range]) |
135.75 (129–140) |
|
Medical history (n/N) |
|
|
Diabetes |
16/20 |
|
Hypertension |
15/20 |
Table 2. Mean serum creatinine, potassium, and sodium at baseline and follow-up visits.
|
Mean (SD) |
Baseline |
6 months |
6-month change from baseline |
12 months |
12-month change from baseline |
|
Serum creatinine (μmol/L) |
120.15 (34.10) |
129.85 (56.80) |
9.70 (42.63) |
129.40 (58.24) |
9.25 (47.34) |
|
Serum potassium (mmol/L) |
4.47 (0.52) |
4.54 (0.34) |
0.08 (0.65) |
4.53 (0.42) |
0.06 (0.66) |
|
Serum sodium (mmol/L) |
135.75 (2.84) |
136.05 (5.09) |
0.30 (4.58) |
136.25 (6.06) |
0.50 (5.20) |
Appendix 1: Mean changes in echocardiogram findings.
|
ECHO parameters |
Mean |
Mean change |
|
|
Before treatment |
After treatment |
||
|
Right ventricular dimension, cm |
3.21 |
3.24 |
0.01 |
|
End-diastolic dimension, cm |
5.4 |
5.42 |
-0.012 |
|
End-systolic dimension, cm |
4.68 |
4.59 |
-0.018 |
|
Interventricular septum, cm |
0.89 |
0.88 |
-0.0053 |
|
Posterior wall thickness, cm |
0.88 |
0.88 |
0.0068 |
|
Left atrium, cm |
4.11 |
4.07 |
-0.0096 |
|
Ejection fraction, % |
25.33 |
27 |
0.079 |
|
Fractional shortening, % |
14.53 |
16.33 |
0.137 |
|
E velocity, cm/sec |
95.86 |
96.46 |
0.031 |
|
A velocity, cm/sec |
57.85 |
61.92 |
0.164 |
|
E/A ratio |
1.95 |
1.64 |
-0.035 |
|
E/e ratio |
21.4 |
19.26 |
-0.098 |
|
Tricuspid annular plane systolic excursion, mm |
15.6 |
15.6 |
0.02 |
|
Longitudinal strain (basal), % |
-11.66 |
-13.93 |
0.21 |
|
Mitral regurgitation grade |
1.4 |
1.33 |
0.047 |
|
Systolic pulmonary artery pressure, mmHg |
46.73 |
42.06 |
-0.078 |
|
Isovolumic relaxation time |
69.33 |
71.33 |
0.053 |
|
Deceleration time, msec |
165.13 |
181.53 |
0.141 |
|
Tricuspid regurgitation severity, msec |
1.4 |
1.53 |
0.107 |
|
Left atrial volume index |
41.53 |
39.6 |
-0.034 |
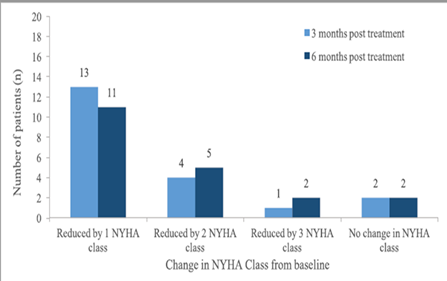
Figure 1. Changes of NYHA class from baseline
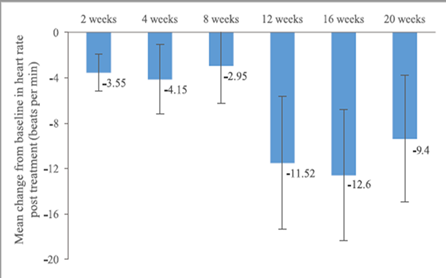
Figure 2. Mean changes in heart rate
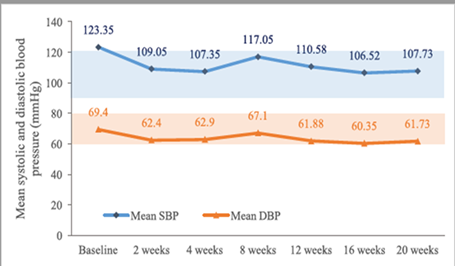
Figure 3. Mean systolic and diastolic blood pressure.
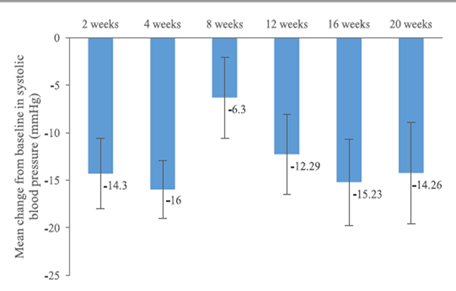
Figure 4. Mean changes of systolic blood pressure from baseline
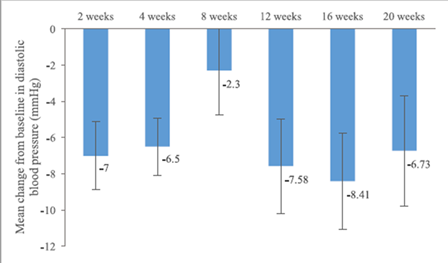
Figure 5. Mean changes of systolic blood pressure from baseline.
