Immunohistochemical Overexpression of nNOS, S100 Protein, and α-Synuclein in Myenteric Plexus of AS/AGU Rats
Muhammad Atteya1*, Aly Mohamed Ahmed1, Raeesa Abdel-Tawab Mohammed1, Musaad Abdulaziz Alfayez1, Shimaa Abdellah Mohammed1, Abdulaziz Siyal1, Tahani Ahmad Al-Matrafi1, Amal AlRabiah1, Hamad Mohammed Alqahtani1, Anthony Payne2
1Department of Anatomy, College of Medicine, King Saud University, Riyadh, Saudi Arabia.
2 Institute of Biomedical and Life Science, Glasgow University, Glasgow, United Kingdom.
*Email: mhasan1@ ksu.edu.sa
ABSTRACT
Objective: There are four currently motor features characterizing Parkinson's disease (PD). These include rigidity of muscles, bradykinesia, tremors at rest, and instability of posture. Along the course of PD, the impairment of motor functions is commonly preceded by nonmotor symptoms (NMS) such as olfactory deficit, difficult swallowing (dysphagia), drooling (sialorrhea), constipation, urinary bladder dysfunction, depression, and sleep disorder. It was suggested that the enteric nervous system could be the initial site for the pathological process leading to PD. Materials and Methods: Six male adult control AS rats (normal control) and six male adults AS/AGU rats (model of PD) were sacrificed. A rectangular strip from the body of the stomach and a cross-section from the duodenum were dissected and processed for histological staining with hematoxylin and eosin, and immunohistochemical staining for detection of nNOS (neuronal NOS), S100 protein (astrocyte marker), and alpha-synuclein (α-synuclein). Results: The histological analysis of the stomach and duodenum of AS/AGU rats demonstrated necrotic smooth muscle cells of muscularis externa. The immunohistochemical analysis of AS/AGU rats showed a statistically significant increase in the expression of nNOS, S100 protein, and α-synuclein expression of myenteric plexuses compared to the control strain AS rats. Conclusion: Gastroduodenal tract of AS/AGU rats showed marked histopathological changes and immunohistochemical overexpression of nNOS, S100, and α-synuclein.
Key words: AS rats, AS/AGU rats, Parkinson’s disease, duodenum, stomach, histology, immunohistochemistry, nNOS, S100, α-synuclein
INTRODUCTION
Parkinson’s disease (PD) is a common disorder of progressive neurodegeneration, characterized by the accumulation of alpha-synuclein (α-synuclein) in neurons of specific brain areas, including the brainstem and cortical regions, and by significant dopaminergic neuron loss [1].
There is proof that glial cells are involved in the beginning and development of PD [2]. Astrocytes have been found to effectively uptake neuronally secreted α-synuclein proteins. Moreover, astrocytes are implicated in inflammatory responses and produce glial inclusions [3]. The distribution of astrocytes positive for α-synuclein coincides with that of Lewy bodies [4]. Also, animal models suggest that microglia are involved early in Parkinson’s disease (PD) [2]. It was proved that specific inhibition of microglial cells led to significant alleviation of the Parkinson’s disease-like symptoms induced by the neurotoxin 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP) [5].
S100, a calcium-binding protein, is synthesized and then secreted by astrocytes [6]. The levels of S100 increase with age and high concentrations of S100 have been detected in many neurodegenerative and neuroinflammatory diseases [7]. It was found that a short time after administration of MPTP the S100-positive astroglia increase [8], which gives rise to the assumption that this protein may also be implicated in the pathogenesis PD. Most interestingly, S100 at low concentrations (nanomolar) acts as a neurotrophic factor promoting neuronal survival and neurite growth during development and under stress conditions [9]. At high concentrations (micromolar), S100 causes neuronal apoptosis by both direct actions on neurons and increasing activation of microglia [6]. These effects may, to some extent, be mediated by activation of inducible nitric oxide synthase (iNOS) [an enzyme responsible for the production of nitric oxide (NO)], and by increasing the level of intracellular calcium inducing caspase-3 activation [10].
The motor impairments that characterize PD are usually preceded, sometimes by up to ten years, by non-motor symptoms (NMS) such as sleep disorder, depression, olfactory deficit, and constipation [11, 12]. Although currently NMS are increasingly associated with PD, they have not yet received considerable attention [11].
Often Patients complain that their NMS is more difficult to manage than their motor problems and may even lead to their hospitalization [13]. Additionally, the alleviation of NMS greatly improves the quality of patients’ lives, especially those who responded to dopaminergic therapy [13]. Therefore, recognition of the presence of NMS early in the course of PD has resulted in a more critical assessment of its etiology and risk factors, and the current developments in PD's neuroprotective and therapeutic biomarkers [14]. As a consequence, PD can no longer only be regarded as a complex motor functional disorder, but as a progressive disease with both motor and non-motor symptoms [13].
Two major neural factors that regulate the gastrointestinal (GI) tract, one is the extrinsic pathway associated with the vagus nerve, and the other is the enteric nervous system (ENS), which is an autonomic nervous system component. The ENS is often known as the human body's second brain because of its ability to operate autonomously independent of the central nervous system (CNS) [15]. The ENS comprises the submucosal (Meisner's) and myenteric (Auerbach's) plexuses, that control the movement and activity of the smooth muscle in the GI tract [16]. The movement function of the GI tract, which is controlled by the ENS, necessitates multiple neurotransmitters including dopamine (DA), acetylcholine, serotonin, vasoactive intestinal peptide (VIP), substance P and nitric oxide synthase (NOS) [17]. While the ENS can operate independently of external stimuli, it communicates closely with the vagal system. [18].
In addition to the endothelial NOS (eNOS) and the inducible NOS (iNOS), neuronal NOS (nNOS) is the NOS isoform in the enteric nervous system. Nitric oxide (NO) is an important neurotransmitter with both non-adrenergic and non-cholinergic effects. It has both a protective and a cytotoxic effect. The protective effect is its retrograde influence over the release of stimulating neurotransmitters and in turn its regulation of neural transduction. The cytotoxic effect of NO is due to its ability to induce cytotoxic free radicals [19].
The smooth muscle layers are innervated by enteric motor neurons, both excitatory and inhibitory, that directly control the motility of the GI tract. Distension of the GI tract by food is detected by the enteric afferent neurons. About 50% of enteric nerves, located in the myenteric plexus that is located between layers of smooth muscles, contain nNOS. NO is the most important inhibitory neurotransmitter in the GI tract [20]. The relaxations of the GI tract and the opening of sphincters are mediated by the inhibitory motor neurons. Increased lower esophageal sphincter relaxations and gastroparesis have been found in mice deficient in nNOS [21].
The main aim of the current study is to investigate the histological changes and immunohistochemical expression of nNOS, S100, and α-synuclein in the stomach and duodenum of a rat model of PD (AS/AGU rat) as a trial to disclose the underlying pathogenesis of gastrointestinal tract symptoms accompanying PD.
MATERIALS AND METHODS
Animals
A total of 12 adult male rats, 6 Albino Swiss (AS) rats (normal control), and 6 AS/AGU rats (model of PD) were used. The rats were obtained from the Experimental Animal Centre in the College of Medicine, King Saud University, Saudi Arabia. The rats were supplied with water and food, ad libitum, at constant humidity and temperature. Under anesthesia, the rats were euthanized by cardiac perfusion with mammalian Ringer’s solution and 4% paraformaldehyde in 0.1 M phosphate buffer. The animals were dissected and a stripe from the body of the stomach and cross-section from the duodenum were excised and stored in 4% paraformaldehyde in phosphate buffer for 48 hours, then embedded in paraffin wax at 57°C. Serial sections were cut at 4 µm and stained for histological and immunohistochemical studies. All experimental procedures have been conducted following the Guidelines for the Care and Use of Laboratory Animals of the College of Medicine Research Center (CMRC) at King Saud University and are in accordance with the Guide to Care and Use of Laboratory Animals published by the National Institutes of Health (NIH).
Histological study
Hematoxylin and eosin (H&E) stain [22] was used to detect any histological changes in the walls of the stomach and duodenum, with special reference to the smooth muscles of the muscularis externa and the myenteric plexuses.
Immunohistochemistry [23]
Immunostaining of the paraffin sections of the stomach and duodenum sections for detection of nNOS, S-100 protein, and α-synuclein was performed using streptavidin-biotinylated horseradish peroxidase (S-ABC) method (Novalink Max Polymer detection system, Novocastra, product No. RE7280-K). Sections were incubated with anti-nNOS (Goat polyclonal, dilution of 1:200) (Cat# abcam-ab5586), anti-S100 antibody (rabbit polyclonal, dilution 1:500) (Cat# abcam-ab868), and anti-α-synuclein antibody (4D6) (mouse monoclonal, dilution 1:500) (Cat# abcam-ab1903) following the datasheets supplied by the manufacturers.
Image analysis
High-resolution whole-slide digital scans of nNOS-, S100-, and α-synuclein-immunostained sections were created with a ScanScope slide scanner (Aperio Technologies, Inc.). The digital slide images were then viewed and analyzed using the viewing and image analysis tools of Aperio’s ImageScope software (Aperio Technologies, Inc.). Five areas, each with a fixed size of 0.066 mm2, were randomly selected per section. To quantify the immunopositive reaction, the color deconvolution (color separation) algorithm (Aperio Technologies, Inc.) was set up to detect and quantify only the brown color of DAB positive staining. The algorithm was then run on the selected area to measure the percentage of immunopositive reaction relative to the total analysis area and the output data were subjected to statistical analysis.
Statistical analysis
Data collected were subjected to statistical analysis using IBM SPSS Statistics, Version 22. The normality and homogeneity of variances of the data were first checked by the Shapiro-Wilk test and Levene’s test, respectively. Analysis of variance (ANOVA) was then used for an overall comparison between the studied groups followed by Scheffe’s test for post hoc pairwise comparisons. Differences were considered significant when p was equal to or less than 0.05.
RESULTS
Histological study
H&E stained sections of the stomach and duodenum from AS rats showed normal histological architecture and well-detectable myenteric plexus located between the circular and the longitudinal layer of the muscularis externa. These plexuses are formed of aggregations of homogenous clusters of ganglion neurons and associated glial cells (Fig. 1 A, C). However, H&E stained sections of stomach and duodenum from AS/AGU rats showed numerous necrotic smooth muscle cells (SMCs) of muscularis externa with pyknotic or karyolitic nuclei. There are patches of SMCs with intensive eosinophilic cytoplasm and pyknotic nuclei, alternating with patches of necrotic SMCs showing karyolitic nuclei and interstitial edema. Myenteric plexuses showed lacunar spaces with necrotic neurons and glial cells (Fig. 1 B, D).
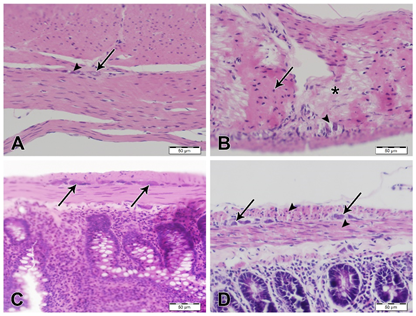
Fig. 1: H&E stained sections of stomach and duodenum, scale bars; 50 µm. (A) Stomach from AS rat showing normal histological features with myenteric plexus showing normal ganglion neurons (arrow) and glial cells (arrowhead). (B) Stomach from AS/AGU rat showing marked interstitial edema in between SMCs of muscularis externa. Numerous muscle cells are necrotic with pyknotic nuclei (arrow) or karyolysis (asterisk). The sarcoplasm of these cells reveals intense eosinophilic staining. Also, neuronal cells of the myenteric plexus are few and show pyknotic nuclei with a marked decrease of glial cells, the plexus also shows vacuolations (arrowhead). (C) Duodenum from AS rat showing normal appearance of SMCs and myenteric plexuses (arrows). (D) Duodenum from AS/AGU rat showing degenerated SMCs (arrowheads) and degenerated neuronal and glial cells of myenteric plexus (arrows).
Immunohistochemistry
nNOS immunostaining showed an intensely strong increase in immunostaining in nerve cells of myenteric plexus of stomach and duodenum of AS/AGU rats compared with AS control rats (Fig. 2).
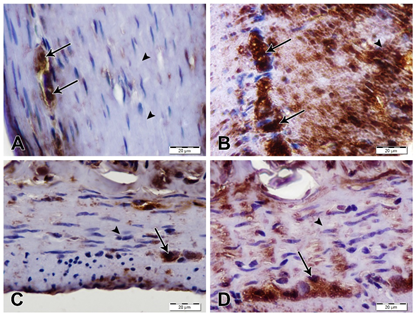
Fig. 2: nNOS immunostained sections of stomach and duodenum, scale bars; 20 µm. (A) Stomach from AS rat showing moderate nNOS immunostaining of nerve cells in myenteric plexus (arrows) while smooth muscles of muscularis externa show negative immunostaining (arrowheads). (B) Stomach from AS/AGU showing strong nNOS immunostaining in nerve cells of myenteric plexus (arrows) as well as the smooth muscles of muscularis externa (arrowhead). (C) Duodenum from AS rat showing moderate nNOS immunostaining in nerve cells of myenteric plexus (arrow) and negative immunostaining in the smooth muscle of muscularis externa (arrowhead). (D) Duodenum from AS/AGU rat showing strong nNOS immunostaining in nerve cells of myenteric plexus (arrow). However, SMCs reveal negative immunostaining (arrowhead).
S100 immunostaining showed a marked increase of immunoreactivity in nuclei and cytoplasm of glial cells of myenteric plexus of stomach and duodenum of AS/AGU rats compared with AS rats (Fig. 3).
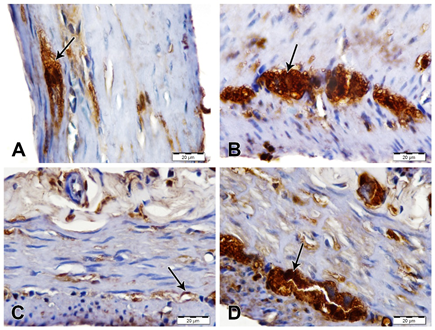
Fig. 3: S100 immunostained sections of stomach and duodenum, scale bars; 20 µm. (A) Stomach from AS rat showing moderate S100 expression in glial cells of myenteric plexus (arrow). (B) Stomach from AS/AGU showing strong S100 immunostaining in glial cells of myenteric plexus (arrow). (C) Duodenum from AS rat showing mild S100 immunostaining (arrow). (D) Duodenum from AS/AGU rat showing strong S100 immunostaining in glial cells of myenteric plexus (arrow).
Alpha-synuclein immunostaining showed a marked increase of immunoreactivity in neurons of myenteric plexus of stomach and duodenum of AS/AGU rats compared with AS rats (Fig. 4).
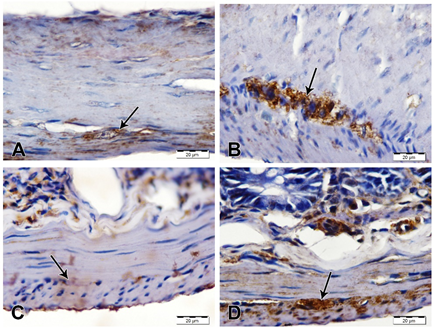
Fig. 4: α-synuclein immunostained sections of stomach and duodenum, scale bars; 20 µm. (A) Stomach from AS rat showing weak α-synuclein immunostaining of nerve cells in myenteric plexus (arrow). (B) Stomach from AS/AGU showing strong α-synuclein immunostaining in nerve cells of myenteric plexus (arrow). (C) Duodenum from AS rat showing negative α-synuclein immunostaining in nerve cells of myenteric plexus (arrow). (D) Duodenum from AS/AGU rat showing strong α-synuclein immunostaining in nerve cells of myenteric plexus (arrow).
Statistical analysis
The results of the statistical analysis are shown in Table 1 and Figs. 5, 6.
Table 1. nNOS, S100, and α-Synuclein expression (area %) in the studied groups.
|
|
Duodenum |
Stomach |
||||
|
|
AS (Mean ± SE) |
AGU (Mean ± SE) |
P-value |
AS (Mean ± SE) |
AGU (Mean ± SE) |
P-value |
|
nNOS |
21.783 ± 3.936 |
52.441 ± 3.380 |
0.000* |
15.496 ± 2.033 |
34.311 ± 2.845 |
0.001* |
|
S100 |
17.50 ± 3.213 |
54.773 ± 1.498 |
0.000* |
17.703 ± 3.190 |
49.353 ± 2.394 |
0.000* |
|
α-Synuclein |
17.385 ± 0.851 |
50.739 ± 3.224 |
0.000* |
18.019 ± 1.638 |
55.990 ± 2.865 |
0.000* |
* significant difference (P ≤ 0.05)
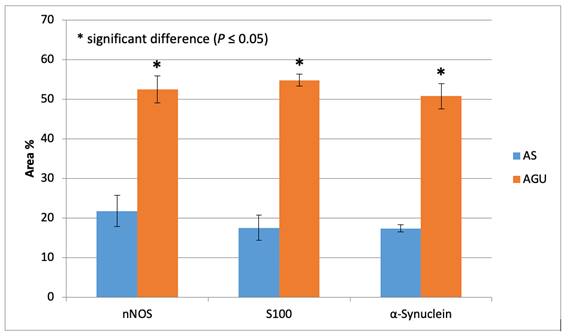
Fig. 5: nNOS, S100, and α-Synuclein expression (area %) in duodenum.
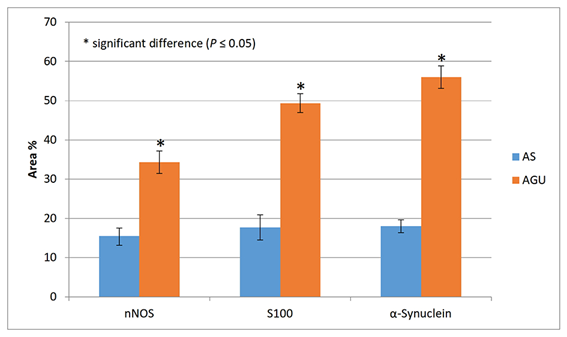
Fig. 6: nNOS, S100, and α-Synuclein expression (area %) in the stomach.
DISCUSSION
Braak et al [24] and Braak et al [25] stated that PD might be initiated by an infection of intestinal and olfactory mucosae. One of our findings in H&E stained sections of PD model (AS/AGU) rats is marked cytoplasmic and nuclear degenerations of smooth muscle fibers of muscularis externa and vacuolation of myenteric plexuses of stomach and duodenum which can be explained by the effect of infections preceding the appearance of symptoms and signs of PD.
However, NMS, which occur before motor symptoms, were accompanied by clear evidence of Lewy pathology, supporting Braak’s concept [26]. Other authors hypothesized that PD pathology could initially develop in the enteric nervous system (ENS) and then spreads to the central nervous system (CNV) [27, 28].
Additionally, a recent study also supporting Braak’s hypothesis indicated that α-synuclein, a part of Lewy bodies, injected into mice intestine can reach the dorsal motor nucleus in the brain stem through the vagus nerve [29].
Early PD is often accompanied by impairments of the autonomic nervous system and GI symptoms represent the most common non-motor symptoms (NMS) [30].
It was reported that the NMS associated with early PD principally includes GI symptoms as a result of the imbalance of the autonomic nervous system. A subpopulation of neurons producing NO has been identified in the gastrointestinal tract [31]. Nitric oxide is considered as an important mediator of smooth muscle relaxation [32]. Its synthesis is catalyzed by nNOS [32]. Also, NO is responsible for repairing the tissue and has a gastrointestinal protective role [33]. However, high levels of NO induce numerous pathological lesions of the gastrointestinal tract (GIT) [33].
In the present study, immunostaining of myenteric plexuses using anti-nNOS primary antibody revealed a significant increase of immunoreactivity which may participate in the degenerative changes of both smooth muscles of muscularis externa and myenteric plexuses. Also, nNOS may act as an early detecting factor leading to PD, according to Braak's hypothesis. Musara and Vaillant [34] stated that staining for nNOS revealed a large component of nitrergic nerves in the circular smooth muscle. This finding supports the growing concept that inhibitory motor control makes an important contribution to normal motor function in the gastrointestinal tract.
Our finding of a significant increase in the nNOS immunoreactivity can explain the GI symptoms preceding PD manifestations. Also, there are other opinions to explain the GI changes in PD [35]. These explanations include neurodegeneration, overexpression of α-synuclein, inflammatory reaction, and hyperpermeability of the intestinal wall as likely pathogenic factors behind GI changes [35]. In accordance with these opinions, the present study showed a marked increase of α-synuclein which acts as an indicator for PD. Furthermore, other pathogenic factors responsible for the induction of PD include the destruction of lysosomes, proteasomes, endoplasmic reticulum and mitochondria, and oxidative stress [35, 36].
One of our important findings is the increase of immunohistochemical expression of S100 in the myenteric plexuses of both stomach and duodenum of AS/AGU rats, which is similar to that discovered in substantia nigra in patient with PD [37].
CONCLUSION
The present study showed marked necrosis of smooth muscle cells of muscularis externa of AS/AG rats, associated with a marked increase of immunoreactivity of nNOS, S-100 protein, and α-synuclein expression in the myenteric plexuses.
Clinical implication
Gastrointestinal changes represent the principal NMS associated with PD and can lead to serious complications including malnutrition, megacolon, and intestinal obstruction. For fundamental progress in the therapy of PD, the peripheral aspects of PD should remain a priority to improve the therapeutic approaches to the disease, which are clearly in need of major improvements.
Limitations of the study
Our study has some limitations such as not performing tyrosine hydroxylase immunohistochemistry to verify the presence of peripheral change of dopamine synthesis as a similar mechanism of PD that happens in substantia nigra of CNS but we believe that α-synuclein immunohistochemistry has the same reliability in this verification. Another limitation of the study is the relatively low number of evaluated animals but that was because of the rarity of the AS/AGU strain. We recommend further studies to be done using different PD animal models to verify our results.
Declaration of Interests
The authors declare no competing interests.
ACKNOWLEDGMENTS
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No. (RGP-1438-030).
REFERENCES
- Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009; 373(9680): 2055-2066.
- Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson's disease. Mov Disord. 2011; 26(1): 6-17.
- Lee HJ, Suk JE, Patrick C, et al. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010; 285(12): 9262-9272.
- Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson's disease. Acta Neuropathol. 2007; 114(3): 231-241.
- Wu DC, Jackson-Lewis V, Vila M, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002; 22(5): 1763-1771.
- Sorci G, Bianchi R, Riuzzi F, et al. S100B Protein, A Damage-Associated Molecular Pattern Protein in the Brain and Heart, and Beyond. Cardiovasc Psychiatry Neurol. 2010; 2010.
- Rothermundt M, Peters M, Prehn JH, et al. S100B in brain damage and neurodegeneration. Microsc Res Tech. 2003; 60(6): 614-632.
- Muramatsu Y, Kurosaki R, Watanabe H, et al. Expression of S-100 protein is related to neuronal damage in MPTP-treated mice. Glia. 2003; 42(3): 307-313.
- Bianchi R, Adami C, Giambanco I, et al. S100B binding to RAGE in microglia stimulates COX-2 expression. J Leukoc Biol. 2007; 81(1): 108-118.
- Iuvone T, Esposito G, De Filippis D, et al. Cannabinoid CB1 receptor stimulation affords neuroprotection in MPTP-induced neurotoxicity by attenuating S100B up-regulation in vitro. J Mol Med (Berl). 2007; 85(12): 1379-1392.
- Sauerbier A, Ray Chaudhuri K. Non-motor symptoms: the core of multi-morbid Parkinson's disease. Br J Hosp Med (Lond). 2014; 75(1): 18-24.
- Pahwa R, Lyons KE. Early diagnosis of Parkinson's disease: recommendations from diagnostic clinical guidelines. Am J Manag Care. 2010; 16 Suppl Implications: S94-99.
- Todorova A, Jenner P, Ray Chaudhuri K. Non-motor Parkinson's: integral to motor Parkinson's, yet often neglected. Pract Neurol. 2014; 14(5): 310-322.
- Berg D, Postuma RB, Bloem B, et al. Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson's disease. Mov Disord. 2014; 29(4): 454-462.
- Marrinan S, Emmanuel AV, Burn DJ. Delayed gastric emptying in Parkinson's disease. Mov Disord. 2014; 29(1): 23-32.
- Derkinderen P, Rouaud T, Lebouvier T, et al. Parkinson disease: the enteric nervous system spills its guts. Neurology. 2011; 77(19): 1761-1767.
- Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. 2004; 16 Suppl 1: 55-59.
- Lebouvier T, Chaumette T, Paillusson S, et al. The second brain and Parkinson's disease. Eur J Neurosci. 2009; 30(5): 735-741.
- Sugata H, Ueno T, Shimosegawa T, et al. Direct detection of nitric oxide and its roles in maintaining gastric mucosal integrity following ethanol-induced injury in rats. Free Radic Res. 2003; 37(2): 159-169.
- Bult H, Boeckxstaens GE, Pelckmans PA, et al. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990; 345(6273): 346-347.
- Mashimo H, Goyal R. Lessons from genetically engineered animal models. IV. Nitric oxide synthase gene knockout mice. Am J Physiol. 1999; 277: G745-G750.
- Drury R, Wallington E (1980) Preparation and fixation of tissues. In: Drury R, Wallington E, editors. Carleton's Histological Technique. 5th ed. Oxford University Press, Oxford, pp. 41-54.
- Ohira T, Saito T, Ando R, et al. Systemic histopathology of infant rats exposed to busulfan. J Toxicol Pathol. 2014; 27(1): 25-29.
- Braak H, Rub U, Gai WP, et al. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm (Vienna). 2003; 110(5): 517-536.
- Braak H, Ghebremedhin E, Rub U, et al. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004; 318(1): 121-134.
- Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009; 8(5): 464-474.
- Phillips RJ, Walter GC, Wilder SL, et al. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson's disease? Neuroscience. 2008; 153(3): 733-750.
- Cersosimo MG, Benarroch EE. Pathological correlates of gastrointestinal dysfunction in Parkinson's disease. Neurobiol Dis. 2012; 46(3): 559-564.
- Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014; 128(6): 805-820.
- Cloud LJ, Greene JG. Gastrointestinal features of Parkinson's disease. Curr Neurol Neurosci Rep. 2011; 11(4): 379-384.
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43(2): 109-142.
- Li CG, Rand MJ. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur J Pharmacol. 1990; 191(3): 303-309.
- Kochar NI, Chandewal AV, Bakal RL, et al. Nitric oxide and the gastrointestinal tract. Int J Pharmacol. 2011; 7: 31-39.
- Musara C, Vaillant C. Immunohistochemical studies of the enteric nervous system and interstitial cells of Cajal in the canine stomach. Onderstepoort J Vet Res. 2013; 80(1): 518.
- Celardo I, Martins LM, Gandhi S. Unravelling mitochondrial pathways to Parkinson's disease. Br J Pharmacol. 2014; 171(8): 1943-1957.
- Smith WW, Jiang H, Pei Z, et al. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet. 2005; 14(24): 3801-3811.
- Sathe K, Maetzler W, Lang JD, et al. S100B is increased in Parkinson's disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-alpha pathway. Brain. 2012; 135(Pt 11): 3336-3347.
