Nanoformulations: Clinoptilolite-Based Capsule with Lecithin Shell
A.G. Pogorelov 1*, T.A. Stepanova 1, A.I. Panait 1, M.A. Pogorelova 1, O.A. Suvorov 1 A.A. Gulin 2, 3
1 Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, 142290, Pushchino, Moscow Region, Russian Federation.
2 N. N. Semenov Institute of Chemical Physics, Russian Academy of Sciences, ul. Kosygina 4, 119991, Moscow, Russian Federation.
3 Moscow State University, Department of Chemistry, GSP-1, Leninskiye Gory 1-3, 119991, Moscow, Russian Federation.
ABSTRACT
Microporous materials can provide interesting tools for different goals from bioimaging to the delivery of bioactive molecules. In this study, the procedure based on cryo-approaches was designed to formulate the nanoparticles of natural clinoptilolite from the Zeolites mineral family. Applying scanning electron microscopy, clinoptilolite samples were imaged. After aging in the ethanol solution of phosphatidylcholine (lecithin), nanoparticles were encapsulated in the lecithin envelope. The adsorption of lecithin by clinoptilolite nanoparticles was studied by registering the diminution of optical density (OD 235) for ethanol/lecithin solution at 235 nm with UV spectrometry. The kinetics of lecithin/clinoptilolite complex development was shown to exhibit intricate behavior, when the adsorption of lecithin was followed with its gradual release resulting in the increase of lecithin content in ethanol solution back toward the original level. The size distribution for the lecithin/clinoptilolite complex was determined with a dynamic light scattering technique. To our knowledge, there are no reports of natural clinoptilolite-based platforms that were used as the for a nanosized capsule with phospholipids shell.
Key words: nanocapsule, licitin, clinoptilolite, UV spectrometry, scanning electron microscopy, particle size measurements.
INTRODUCTION
Oral delivery is the most common method for biologically active molecule administration in a clinical procedure in humans. This approach is especially effective to deliver substances directly to the enteric epithelium [1]. Microorganisms represent excellent sources of enzymes [2]. However, the enzymatic activity, and the acidic gastric environment, and the continuous mucus secretion can significantly limit the therapeutic effect. To defend specific activity against the aggressive environment of the gastrointestinal tract, the administrated inorganic or solid substances are engineered into nanoscale dispersive formulations [3-7].
The applications of nanotechnology are exponentially growing in industry and medicine, due to interesting properties of engineered nanoparticles [8]. Nanoparticles offer distinct properties such as particle size, increased chemical reactivity, and increased surface area/mass ratio compared to their bulk counterparts [9]. Nanomaterials in the life cycle and ecosystems exhibit the lowest toxicity [10]. Porous materials are considered as promising materials for creating the micro and nanocontainers [11–13]. Zeolite is one of the most abundant natural mineral widely distributed throughout the world, and used in consumer products, and utilized in agriculture [14-16]. The microporous structure significantly increases the surface area of zeolite particles available for adsorption. Among the naturally occurring zeolites, clinoptilolite is the most widespread and studied for the application in biomedical engineering [17]. This mineral acts like an antioxidant [18], and anti-inflammatory agents [19], and excellent detoxifying [20]. A method has been developed to obtain synthetic zeolite nanoparticles in laboratory conditions [21–26]. Even though there are several synthetic species of zeolite, their production is limited by small volumes, the cost of final products, and the need to purify it from ingredients involved in the synthesis. An alternative to synthetic nanocrystals seems to be nanoparticles from natural zeolites, which are mined in an industrial manner [16, 27].
To reach enteric epithelium, orally administrated nanoparticles must overcome numerous hurdles. Among them, poor bioavailability and low solubility make penetration through the mucus barrier challenge. The nanoparticle surface can be modified to enhance mucoadhesion for targeting the mucus layers that protect the apical membrane of enterocytes. The coating of particles with shell allows the biocompatibility of water-insoluble inorganic [28-30] or solid [31, 32] materials. For this purpose, the surface of nanoparticles can be improved with phospholipids [33, 34]. Thereby, the composition of zeolite (platform) and phospholipid (shell) is considered as the promising complex for formulating the nanocapsules.
An example of the successful use of aluminosilicate for the transport of biomolecules is a study in which a synthetic zeolite-L nanocrystal was employed as a container for the delivery of nucleic acids and organic molecules [28]. In this work, a poly-L-lysine coating permits the transport of nanoparticles into HeLa cells to be more efficient. However, to the best of our knowledge, there are no reports of natural zeolite nanoparticles with phospholipids shell. Herein we aim: (i) to develop a low-cost scheme for the preparation of nanosized particles from natural clinoptilolite in task laboratory without the high energy input and mill units, (ii) to study the adsorption of phosphatidylcholine by the particles obtained.
Materials and Methods
Pretreatment of clinoptilolite. Natural zeolite originated from the Kholinsky deposit, Russia was used in this study. Commercially available product Litovit M (Russia) was the source of powdered homogeneous clinoptilolite containing 100% for the activated component. To achieve the microsized particles, dry clinoptilolite powder was triturated in a porcelain mortar at room temperature.
Scanning electron microscopy. The investigated samples were visualized by scanning electron microscopy. The suspension of clinoptilolite particles in distilled water (30 mg/100 ml) was pretreated with ultrasound for 40 seconds. The suspension droplet of ~ 2 μl was placed on the surface of the sample holder for an electron microscope. After the water was air-dried at room temperature, specimens were platinum-coated using a sputter coater JFC-1600 (JEOL, Japan). A Pt film significantly enhances the signal of secondary electrons, removes the electrostatic charge, and protects the sample from heating, which causes its mechanical destruction. The fine structure of the object relief was studied in a scanning electron microscope JSM-6390A (JEOL, Japan) at an accelerating voltage of 25 kV in the secondary electron mode.
Extraction of phosphatidylcholine. Phosphatidylcholine (lecithin) was extracted from the nutrition additive of E322 (Cargill Lecigran 1000P, Germany) consisting of the composition of polar phospholipids. Briefly, 600 mg of commercially available E322 was mixed with 6 ml of 100% Ethyl Alcohol (ETOH) until a homogeneous suspension. The mixture was then incubated for 24 hours at room temperature under gentle magnetic stirring. Then, the undissolved components were separated by centrifugation at 600g for 10 minutes. The obtained clear stock solution (2.4 wt%) was sealed in a glass vial and was stored at 4°C in the dark. For the working medium, the stock solution was diluted with ETOH in the ratio of 1:30 ([stock solution]: ETOH).
Complex formation between clinoptilolite and lecinit. Clinoptilolite/lecinit complex was spontaneously formed upon incubation of nanoparticles (20 mg) in the working medium (4 ml) at room temperature for intervals (1/4, 1/2, 1, 1.5, 2, 3, 4 and 6 h) on a horizontal shaker. After the suspension was centrifuged at 600 g for 10 min, the supernatant (3 ml) was withdrawn and it was investigated by UV spectrometry.
UV spectrometry at 235 nm. In vitro adsorption of lecithin with nanoparticles was inspected using the characteristic peak of 235 nm that has been attributed to carbonyl groups. The value of optical density (OD 235) for the working medium was 0.76 which corresponds to optimal conditions for analytical investigations carrying out with a Specord M40 spectrophotometer (Carl Zeiss, Germany).
Results and discussion
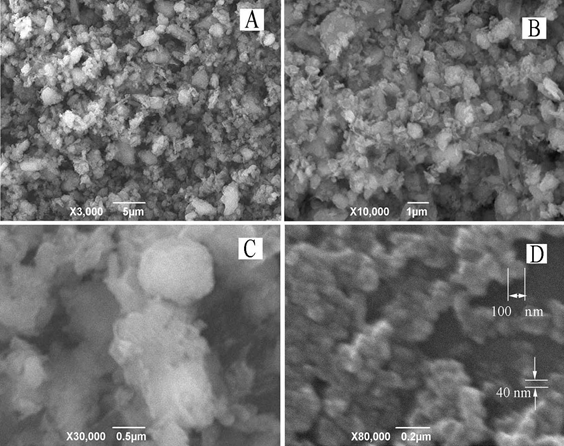
Nanoscale particles of natural clinoptilolite. Clinoptilolite samples were visualized by scanning electron microscopy. Figure 1 shows the set of micrographs demonstrating the size and shape of clinoptilolite particles at different stages of the proposed procedure.
|
|
Figure 1. Micrographs of clinoptilolite particles: (A) commercially available product “Litovit M”; (B) microsized particles after grinding “Litovit M” in a porcelain mortar at room temperature; (C) aggregations of nanosized particles originated after cold powder contacts immediately with the atmosphere humidity; (D) nanosized particles after the cold powder was previously warmed in a vacuum chamber. Images were obtained with scanning electron microscopy in the mode of secondary electrons.
It is seen that the pretreatment of “Litovit M” (Fig. 1A) in a porcelain mortar permits microsized particles to be prepared (Fig. 1B). To reach nanoscale sizes, clinoptilolite powder was ground in a cryo box at -35°C using an agate mortar. Note that cold samples had to avoid contact with atmosphere humidity resulting in the condensation of water droplets followed by the aggregation of nanoparticles (Fig. 1C). Therefore, after cryogrinding, the cold sample of powdered clinoptilolite was transferred under the vapor of liquid nitrogen to a vacuum chamber, where it was warmed gradually to room temperature (Fig. 1D).
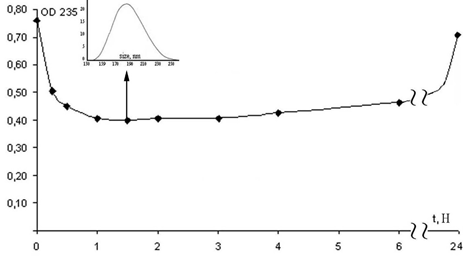
Clinoptilolite/lecithin complex. Complex formation was recorded with UV spectrometry (Fig. 2), where changes in optical density imply the alteration of lecithin concentration in the ETOH solution.
|
|
Figure 2. Changes of optical density (OD 235) in lecithin solution depending on incubation intervals (hours) of clinoptilolite nanoparticles, where the initial value of 0.76 corresponds to the optical density of the working medium at 235 nm. Insertion (indicated by arrow): the size distribution of clinoptilolite/lecithin complexes formed after 1.5 hours of interaction between nanoparticles and lecithin.
The kinetics of lecithin/clinoptilolite complex development was shown to exhibit intricate behavior (Fig. 2). During approximately two hours, the optical density of the lecithin solution reaches the minimum level of 0.4, meaning the maximal adsorption of lecithin on the surface of the nanoparticles. Consequently, the lecithin layer covers the nanosized clinoptilolite core, forming a nanoscale capsule. The insertion (Fig. 2) shows that the sizes of the clinoptilolite/lecithin complex vary from 140 nm to 260 nm with a maximum of 190 nm. However, the adsorption was a reversible process that was followed with the lecithin gradual release resulting in its increase in ETOH solution back toward the original level.
Prerequisites for the successful diffusion of particles through the barrier layer of mucus are nanoscale sizes in the combination with mucoadhesion [35, 36]. Formulating the clinoptilolite-based nanocapsule with lecithin shell, we obey these criteria. Thus, like synthetic zeolite nanocrystals, natural clinoptilolite nanoparticles seem to be designed as carriers for the delivery of bioactive substances. However, they can be applied not only as transporters but for miscellaneous actions like the excellent detoxifying [20], radical scavenging [18], and anti-inflammatory agents [19].
Conclusion
This work describes the first example of the use of natural zeolite to create a nano-platform with phospholipids shell. Nanoscale particles were successfully formulated using ordinary laboratory types of equipment without the high energy input and mill units. The mode size of ~100 nm was achieved after powdering the commercially available product at a low temperature of -35ºC, which was followed with the warming of cold clinoptilolite powder in a vacuum chamber. Thus, cryo-approaches are very promising considering the low-cost scheme for the grinding of tailored nanoproducts. It was shown that the lecithin/clinoptilolite complex development had complicated kinetics. After 2 h of incubation, the initial adsorption of lecithin by clinoptilolite particles has been followed with its very slow release in the ETOH solution. In summary, here, we developed a novel hybrid nanocapsule consisting of the clinoptilolite core and lecithin shell. This result is, therefore, opening an interesting manner to deliver lecithin soluble and adhesive substances into mucus layers.
ACKNOWLEDGMENTS
This work was supported by state task for ITEB RAS № 075-00845-20-00 and, in the part of the development of SEM techniques for bioorganic sample imaging, by the Russian Science Foundation grant № 20-16-00019.
Conflict of interest statement
The authors declare no conflict of interest.
REFERENCES
- Simonoska-Crcarevska, M., Glavas-Dodov, M., Goracinova, K., Chitosan coated Ca–alginate microparticles loaded with budesonide for delivery to the inflamed colonic mucosa, Eur. J. Pharmaceutics and Biopharmaceutics, 2008, 68, 565–578.
- Ahmad MS, AbdEl-Salam BA, Yaser MM, Taha SS. Optimization and characterization of bacterial proteinase enzyme using whey as a fermentation medium. J. Adv. Pharm. Educ. Res 2018;8(2):63-76.
- Render, D., Samuel, T., King, H., Vig, M., Jeelani, S., Babu, R.J., Rangari, V., 2016. Biomaterial-Derived Calcium Carbonate Nanoparticles for Enteric Drug Delivery, J. Nanomaterials, 2016, 2016, 1-8.
- Allemann, E., Leroux, J.-C., Gurney, R., Polymeric nano- and microparticles for the oral delivery of peptides and peptidomimetics, Advanced Drug Delivery Rev., 1998, 34, 171 –189.
- Hu, Y., Jiang, X., Ding, Y., Ge, H., Yuan, Y., Yang, C., Synthesis and characterization of chitosan–poly(acrylic acid) Nanoparticles, Biomaterials, 2002, 23, 3193–3201.
- Jintapattanakit, A., Junyaprasert, V.B., Maob, S., Sitterberg, J., Bakowsky, U., Kissel, T., Peroral delivery of insulin using chitosan derivatives: A comparative study of polyelectrolyte nano-complexes and nanoparticles, Int. J. Pharmaceutics, 2007, 342, 240–249.
- Zhu, J., Hayward, R.C., Hierarchically Structured Microparticles Formed by Interfacial Instabilities of Emulsion Droplets Containing Amphiphilic Block Copolymers, Angew Chem. Int. Ed., 2008, 47, 2113 –2116.
- Haripriya S, Ajitha P. Antimicrobial efficacy of silver nanoparticles of Aloe vera. J. Adv. Pharm. Educ. Res 2017;7(2):163-167.
- Babaei H, Sepahy AA, Amini K, Saadatmand S. The Effect of Titanium Dioxide Nanoparticles Synthesized by Bacillus tequilensis on clb Gene Expression of Colorectal Cancer-causing Escherichia coli. Arch. Pharm. Pract. 2020;11(1):22-31.
- Ghorbani M, Baharara J, Eidi A, Namvar F. Green Biosynthesis of ZnO Nano-Particles, Inhibited Development of Pre-antral Follicles. Arch. Pharm. Pract. 2019;10(1):38-49.
- Yu, X., Khalil, A., Dang, P.N., Alsberg, E., Murphy, W.L., Multilayered Inorganic Microparticles for Tunable Dual Growth Factor Delivery, Adv. Funct. Mater., 2014, 24, 3082–3093.
- Kar, S., An Overview of Recent Advances in Application of Some Inorganic Materials-Biological and Technological Perspectives, J. Biotechnol. Biomater., 2016, 6(3), 565–578.
- Stefanache, A., Ignat, M., Peptu, C.A., Diaconu, A., Stoleriu, I, Ochiuz, L., Development of a Prolonged-Release Drug Delivery System with Magnolol Loaded in Amino-Functionalized Mesoporous Silica, Appl. Sci., 2017, 7, 237-250.
- Kojića, D., Pajevića, S., Jovanović-Galovića, A., Puraća, J., E., Škondrićb, S., Milovaca, S., Popovića, Ž., Grubor-Lajšić, G., Efficacy of natural aluminosilicates in moderating drought effects on the morphological and physiological parameters of maize plants (Zea mays l.), J. Soil Sci. and Plant Nutrition, 2012, 12(1), 113-123.
- Yada, R.Y., Buck, N., Canady, R., DeMerlis, C., Duncan, T., Janer, G., Juneja, L., Lin, M., McClements, D.J., Noonan, G., Oxley, J., Sabliov, Cr., Tsytsikova, L., Vázquez-Campos, S., Yourick, J., Zhong, Q., Thurmond, S., Engineered Nanoscale Food Ingredients: Evaluation of Current Knowledge on Material Characteristics Relevant to Uptake from the Gastrointestinal Tract, Food Science and Food Safety, 2014, 13(4), 730–744.
- Bohacs, K., Faitli, J., Bokanyi, L., Mucsi, G., Control of Natural Zeolite Properties by Mechanical Activation in Stirred Media Mill, Arch. Metall. Mater., 2017, 62(2B), 1399-1406.
- Mastinu, A., Kumar, A., Maccarinelli, G., Bonini, S.A., Premoli, M., Aria F., Gianoncelli A., Memo M., Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral, Molecules, 2019, 24, 1517-1531.
- Pellegrino, P., Mallet, B., Delliaux, S., Jammes, Y., Guieu, R., Schaf, O., Zeolites are effective ROS-scavengers in vitro, Biochemical and Biophysical Research Communications, 2011, 410, 478–483.
- Bacakova, L., Vandrovcova, M., Kopova, I., Jirka I., Applications of zeolites in biotechnology and medicine – a review, Biomater. Sci., 2018, 6(5), 974-989.
- Selim, M.M., El-Mekkawi, D.M., Aboelenin, R.M.M., Sohair, A.S.A., Ghada, M.M., Preparation and characterization of Na-A zeolite from aluminum scrub and commercial sodium silicate for the removal of Cd2+ from water, J. Association of Arab Universities for Basic and Applied Sciences, 2017, 24(1), 19-25.
- Johnson, E.B.G., Arshad, S.E., Hydrothermally synthesized zeolites based on kaolinite: A review, Appl. Clay Sci., 2014, 98, 215–2
- Severance, M.A. Nanocrystalline Zeolites: Synthesis, Mechanism, and Applications. Dissertation for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University; 2014.
- Tian, Y. Synthesis and Characterization of Crystalline Microporous Materials: Investigation of New Synthetic Routes. A Thesis for the Degree of Ph.D. at the University of St Andrews; 2014.
- Mintova, S., Grand, J., Valtchev, V., Nanosized zeolites: Quo Vadis?, C. R. Chimie, 2016, 19, 183-191.
- Tiburu, E.K., Salifu, A., Aidoo, E.O., Fleischer, H.N.A., Manu, G., Yaya, A., Zhou, H., Efavi, J.K., Formation of Chitosan Nanoparticles Using Deacetylated Chitin Isolated from Freshwater Algae and Locally Synthesized Zeolite A and their Influence on Cancer Cell Growth, J. Nano Research, 2016, 48, 156-170.
- Tosheva, L., Belkhair, S., Gackowski, M., Malic, S., Al-Shanti, N., Verran, J., Rapid screening of the antimicrobial efficacy of Ag zeolites, Colloids and Surfaces B: Biointerfaces, 2017, 157, 254–260.
- Elhassan, A.M., Determination of the Natural Zeolites Characteristics in Sudan Country, Int. J. Current Advanced Res., 2016, 5(10), 1304-1310.
- Bertucci, A., Lülf, H., Septiadi, D., Manicardi, A., Corradini, R., De Cola, L., Intracellular Delivery of Peptide Nucleic Acid and Organic Molecules Using Zeolite-L Nanocrystals, Adv. Healthcare Mater, 2014, 3(1), 1812-1817.
- Li, Le-Le, Zhang, R., Yin, L., Zheng, K., Qin, W., Selvin, P.R., Lu, Y., Biomimetic Surface Engineering of Lanthanide-Doped Upconversion Nanoparticles as Versatile Bioprobes, Angew Chem. Int. Ed. Engl., 2012, 51(25), 6121–6125.
- Euliss, L.E., DuPont, J.A., Gratton, S., DeSimone, J., Imparting size, shape, and composition control of materials for nanomedicine, Chem. Soc. Rev., 2006, 35, 1095–1104.
- Grenha, A., Remunan-Lopez, C., Carvalho, E.L.S., Seijo, B., Microspheres containing lipid/chitosan nanoparticles complexes for pulmonary delivery of therapeutic proteins, Eur. J. Pharmaceutics and Biopharmaceutics, 2008, 69, 83–93.
- Shchukina, E.M., Shchukin, D.G., Layer-by-layer coated emulsion microparticles as storage and delivery tool, Current Opinion in Colloid & Interface Science, 2012, 17, 281–289.
- Fricker, G., Kromp, T., Wendel, A., Blume, A., Zirkel, J., Rebmann, H., Setzer, C., Quinkert, R., Martin, F., Müller-Goyman, C., Phospholipids and Lipid-Based Formulations in Oral Drug Delivery, Pharm. Res., 2010, 27, 1469–1486.
- Baldassarre, F., Allegretti, C., Tessaro, D., Carata, E., Citti, C., Vergaro, V., Nobile, C., Cannazza, G., D’Arrigo, P., Mele, A., Dini, L., Ciccarella, G., Biocatalytic Synthesis of Phospholipids and Their Application as Coating Agents for CaCO3 Nanocrystals: Characterization and Intracellular Localization Analysis, ChemistrySelect, 2016, 1(20), 6507–6514.
- Ensign, L.M., Cone, R., Hanes, J., Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers, Advanced Drug Delivery Reviews, 2012, 64, 557–570.
- Kotta, S., Wadood Khan, A., Pramod, K., Ansari, S.H., Sharma, R.K., Ali, J., Exploring oral nanoemulsions for bioavailability enhancement of poorly water-soluble drugs, Expert Opin. Drug Deliv., 2012, 9(5), 585-598.