A Mini Review Study on Mitochondria and Citrate Synthase
Shahad Alzahrani1, Waad Alharbi1, Huda Khawaji1, Sahar Elashmony2,3, Yosra Alhindi4*
1Faculty of Pharmacy, Umm Al-Qura University, Makkah, KSA.
2Department of Pharmacology and Toxicology, Faculty of Medicine, Umm Al-Qura University, Al-Qunfudah, KSA.
3Department of Pharmacology, Faculty of Medicine, Cairo University, Cairo, Egypt.
4Department of Pharmacology and Toxicology, Faculty of Medicine, Umm Al-Qura University, Makkah, KSA.
*Email: [email protected]
ABSTRACT
The regulation of energy production characterizes mitochondrial respiration. A major cycle conquers inside the mitochondria, which regulates this process. This cycle is called the tricarboxylic acid cycle. Citrate synthase is an essential marker of mitochondria. Moreover, it works as a key enzyme in many processes inside the mitochondria. It utilizes the acetyl-CoA and oxaloacetate that participate in energy production inside the mitochondria. Mitochondria known as the powerhouse of the cell. However, their function is extending beyond the energy production. They are very well known for cellular, enzymatic and metabolism pathway. Moreover, mitochondria play a vital role in the function of skeletal muscle and metabolism. Insulin resistance is somehow considered related to an imbalance in mitochondria oxidative capacity. The coordination between citrate synthase and mitochondria has been studies in many labs and research were done but still the exact mechanisms is still unclear. This mini-review elucidates the vital information about mitochondria, citrate synthase, and their relation to obesity and insulin resistance.
Key words: Citrate synthase, Metabolism, Kerbs cycle, Energy, Insulin resistance
INTRODUCTION
Mitochondria and the tricarboxylic cycle
The tricarboxylic acid (TCA) cycle, also known as the citric acid cycle or the Krebs cycle, is a major glucose oxidation pathway that produces NADH and FADH2 through respiration for ATP production (Figure 1) [1]. Moreover, it plays a central role in substrate metabolism by regulating the production of energy in mitochondrial respiration [2]. The TCA cycle is efficient because it provides a large amount of ATP compared to anaerobic glycolysis [2]. The metabolites of the TCA cycle were considered byproducts of cellular metabolism important for the biosynthesis of macromolecules like lipids, proteins, and nucleotides [3]. Studies showed that mitochondria can control cell fate and function by releasing TCA (tricarboxylic acid) cycle metabolites [3]. Regarding obesity, it is unknown whether highly obese people have less TCA cycle flux in their muscles, which might then partition glucose toward the production of glycolytic products [1]. However, there are certain elements of the TCA cycle and chemical pathways are still not well known. Like the influence of mitochondrial enzymes, for example, CS activity, on the metabolism of FA and carbohydrates [2].
|
|
|
Figure 1. TCA cycle |
Cytosolic citrate and the citrate synthase
Citrate synthase (CS) is a marker of mitochondrial function, and it is a key enzyme of the Krebs cycle, which occurs in the mitochondria only. Endurance exercise training can increase CS activity. A recent study showed that CS activity may influence metabolic performance and fitness. Despite that, it is proved that too high CS activity may negatively affect energy metabolism. For example, following a meal, mitochondrial FA oxidation is inhibited by glucose oxidation. It seems that large quantities of cytosolic citrate produced by high CS activity may indirectly block the metabolism of lipids, resulting in insulin resistance and obesity. As a result, the Cs gene (which codes for the CS protein) may be a potential drug target for the treatment of obesity. Nuclear DNA encodes the mitochondrial CS, which is a precursor translationally transcribed in the cytoplasm. Following synthesis, CS is transferred to the mitochondria's inner membrane. Various substrates, including ATP, tricarboxylic acids, and acyl-CoA, can influence CS activity. Acetyl-CoA and oxaloacetate are condensed by CS to produce citrate. It is generally accepted that CS, which catalyzes the first reaction of the TCA cycle by condensation of acetyl-CoA with oxaloacetate to form citrate, is the cycle's rate-limiting enzyme. Acetyl-CoA and OAA are converted into citryl-CoA (Cit-CoA) by a reversible condensation process, which is followed by an irreversible thioester hydrolysis to produce citrate and CoA. Additionally, CS is crucial for cell growth. Meiosis and cell division abnormalities are facilitated by metabolic dysfunction, which is induced by the downregulation of CS [2].
AMPK and energy sensor
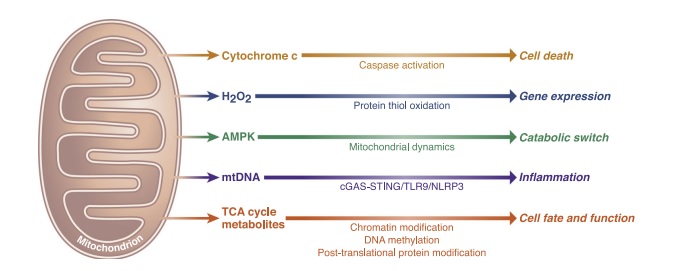
Mitochondria can interact with the rest of the cell via four mechanisms: by releasing cytochrome c to cause cell death, by activating AMP-activated protein kinase (AMPK) to regulate mitochondrial fission and fusion, by producing reactive oxygen species (ROS) to trigger transcription factors, and by releasing mitochondrial DNA (mtDNA) to trigger immunological responses (Figure 2) [3].
|
|
|
Figure 2. Essential signaling functions of mitochondria. |
The primary cellular energy sensor is MP-activated protein kinase (AMPK). When AMPK is activated once cellular energy reserves are depleted, it will increase energy production while decreasing energy waste to restore energy homeostasis. The AMPK pathway will, through neural networks, integrate peripheral signals (mostly hormones and metabolites) at a central level. Additionally, it takes part in hepatic, muscular, and carbohydrate metabolisms as well as other metabolic processes. Numerous anti-obesities and anti-diabetic medications, including nicotine, metformin, and liraglutide, are known to work by centrally or peripherally modulating the AMPK pathway [4].
Mitochondria and apoptosis
Mitochondria are one of the major ancient endomembrane systems in eukaryotic cells. Mitochondria are membrane-bound cell organelles that produce the majority of the chemical energy required to power the cell's biochemical reactions. The mitochondrial energy is stored in a small molecule known as adenosine triphosphate (ATP) [5]. Apoptosis is the process by which cells are programmed to die. It is used to eliminate unwanted cells during early development. Mitochondrial outer membrane permeabilization (MOMP) is a key checkpoint in apoptosis that activates the caspase cascade and causes the majority of cells to die irreversibly. The Bcl-2 family of proteins are apoptotic master regulators that create a complex interaction network within the mitochondrial membrane, affecting MOMP activation [6].
Obesity
Obesity prevalence has increased globally over the last 50 years, reaching pandemic levels. Obesity, commonly described as an excess of body fat that impairs health, is typically measured in clinical practice using the body mass index (BMI), Which is expressed as the ratio of body weight in kilograms divided by height in square meters (kg/m2). It is most often defined as a BMI≥30 [6]. Obesity is a major public health problem; it's associated with increased morbidity and mortality because it significantly increases the risk of diseases such as type 2 diabetes, fatty liver disease, hypertension, myocardial infarction, stroke, dementia, osteoarthritis, obstructive sleep apnea, and several cancers, all of which contribute to a decline in both quality of life and life expectancy [7].
Factors influencing obesity: Obesity is a heterogeneous group of conditions with multiple causes rather than a single disorder. The interaction of genetic, environmental, and psychosocial factors acting through the physiological mediators of energy intake and expenditure determines body weight.
Genetics: Genetic causes of obesity can be broadly classified into 1. Monogenic causes: those caused by a single gene mutation, primarily located in the leptin-melanocortin pathway. 2. Syndromic obesity: severe obesity associated with other phenotypes such as neurodevelopmental abnormalities and other organ/system malformations. 3. Polygenic obesity: caused by the cumulative contribution of many genes whose effect is amplified in a 'weight gain promoting' environment.
Environmental and psychological factors: Lifestyle can affect body weight obesity may be associated with less moderate to vigorous physical activity and more television watching but does not appear to be associated with the amount of sedentary time per day, increased exposure to takeaway food outlets associated with higher obesity rates. Higher stress levels are reported to hinder healthy eating and physical activity, potentially increasing the risk of obesity. Sleep behavior can also affect body weight; short sleep duration is associated with an increased risk of future obesity [8]. Depression is associated with an increased risk of developing long-term obesity, and numerous psychiatric medications are associated with an increased risk of weight gain, including neuroleptics and antidepressants [9].
Metabolic syndrome and insulin resistance
The risk of developing type 2 diabetes and cardiovascular diseases is significantly increased with obesity. Metabolic syndrome refers to a group of metabolic risk factors that are frequently found together and are related to both cardiovascular disease and type 2 diabetes mellitus. These factors include high blood pressure, atherogenic dyslipidemia, insulin resistance, and central obesity [10, 11]. It is most common in overweight and obese patients. The risk factors include smoking, physical inactivity, and family history. The complications include atherosclerotic cardiovascular disease (ASCVD), diabetes, and chronic kidney disease [12].
Insulin signaling and type 2 diabetes
Major risk factors for the development of type 2 diabetes mellitus (T2DM) include age, lifestyle, and diet. The fluidity and permeability of cell membranes, as well as the translocation of glucose transporters and insulin receptor binding and signaling, are all influenced by fatty acids (FAs). Thus, it is hypothesized that FAs may play a crucial part in the generation of insulin resistance (IR) and type 2 diabetes (T2DM). Particular fatty acid (FA) combinations found in phospholipids and triglycerides were found to have the highest correlations with the risk of type 2 diabetes (T2DM) [13].
In the etiology of type 2 diabetes, insulin resistance is one of the earliest issues. Increased nutrient uptake and storage, as well as the inhibition of catabolic processes, are impacts of insulin binding to its receptor in typical target tissues. Studies on human-induced pluripotent stem cells and tissues have shown that phosphorylation and gene expression can alter both inside and outside the typical insulin signaling pathway, leading to cell-autonomous modifications in signaling super-networks. By comprehending how these multi-layered molecular networks control insulin action and metabolism in numerous tissues, it will be possible to treat and prevent type 2 diabetes and its companion disorders [14].
It has been acknowledged that oxidants, particularly H2O2, mimic insulin. Insulin therapy causes cells' intracellular H2O2 levels to rise, influencing the function of downstream signaling elements and amplifying insulin-mediated signal transduction.
Insulin-stimulated H2O2 has specific molecular targets like phosphatases and kinases, whose activity can be changed by redox alterations of essential cysteine residues.
The effects of a number of these redox-sensitive cysteines on insulin signaling have been studied during the past few decades [15].
Obesity and oxidative stress
The prevalence of obesity has been constantly growing both in the United States (US) and around the world. Food addiction is significantly influenced by oxidative stress, which also contributes to and mediates obesity. Adipogenesis is directly influenced by reactive oxygen species, and oxidative stress affects every aspect of obesity, such as genetics, sleep, gut microbiome, insulin, ghrelin, inflammation, adipokines, leptin, stress, and HPA. An antioxidant and prooxidant dietary ratio of 2:3 for each meal is the ideal nutritional ratio for excellent health and ideal weight since redox homeostasis is slanted towards increased reactive oxygen species generation, and excessive antioxidant consumption might cause oxidative stres [16].
Obesity, characterized by excessive fat deposition, is a dangerous condition. Research suggests that oxidative stress may be linked to obesity and its problems. By numerous biological processes, obesity raises oxidative stress. Oxidative stress aids in the growth of obesity by enhancing the storage of fat tissue. By modifying oxidative stress and antioxidant enhancers that minimize endothelial dysfunction and inflammation, exercise-induced weight reduction may enhance redox status [17]. Obesity and overweight are emerging as major public health problems, especially in developing countries. Women of childbearing age have a higher prevalence of these health problems. Chronic oxidative stress is a hallmark of overweight and obesity, harming both mothers and children. We, therefore, investigate whether maternal body mass index before pregnancy and nutritional characteristics during pregnancy are associated with increased mother and infant oxidative stress indicators. At the end of the third trimester of pregnancy, the obese mothers' plasma showed increased malondialdehyde and nitric oxide levels than the healthy non-obese mothers. Eating fruits and vegetables was associated with lower levels of NO and MDA in pregnant women (and neonates) and vice versa [18].
Sarcopenic obesity (SO), which combines sarcopenia and obesity, is most common in older individuals. Therefore, in addition to muscle mass and the signaling pathways that control it, oxidative stress in sarcopenic (SO) obesity impairs satellite cell, mitochondrial, and endoplasmic reticulum function [19]. Male infertility, as well as obesity, metabolic syndrome, and type 2 diabetes mellitus, have all been linked to oxidative stress. For controlling oxidative stress, no precise recommendations exist [20].
Nowadays, it is recognized that obesity is a major health problem with several problems that harm the quality of life. It is a multifactorial chronic disease. Extensive research has shown that oxidative stress is a critical relationship between obesity and its associated consequences. Obesity causes metabolic syndrome by increasing oxidative stress in the body and reducing adipokine production. In the process of detoxifying xenobiotic substances, glutathione (GSH), one of the antioxidants, fights free radicals and reactive oxygen species (ROS) [21].
Lipid metabolism and citrate synthase
By producing acetyl-CoA from citrate for fatty acid and cholesterol biosynthesis, ATP citrate lyase (ACLY) is a crucial enzyme that connects carbohydrates to lipid metabolism. A first-of-its-kind, prodrug-based direct competitive inhibitor of ACLY, bempedoic acid (ETC-1002) controls lipid metabolism by enhancing the expression and activation of the hepatic LDL receptor (LDLr) [22].
In healthy cells, the enzyme ATP citrate lyase (ACLY) catalyzes the conversion of citrate to acetyl-CoA, which speeds up the production of new fatty acids. Different tumor forms, including brain, breast, rectal, and ovarian cancers, have been found to collect lipids and fatty acids, which serve as an important source of energy for the growth and metabolism of cancer cells. Since the basis for fatty acid synthesis is the conversion of citrate to acetyl-CoA by the ACLY, ACLY appears to be a relatively undiscovered and promising therapeutic target for anticancer [23]. As the primary producer of acetyl-Coenzyme A, a crucial building block for the production of fatty acids, cholesterol, and isoprenoids, and a component of protein acetylation, ATP citrate lyase (ACLY) is a crucial enzyme in cellular metabolism. They found that additional research has demonstrated a strong link between ACLY and the development of cancer since an increase in lipid synthesis supplies the necessary building blocks for cell growth and division [24].
CONCLUSION
Citrate synthase is an enzyme that is very active in all available cells. Responsible mainly for catalyzing the first reaction of the Kerbs cycle. Research has shown that citrate synthase can be associated with diabetes and obesity; however, the exact mechanisms are still not very clear. Lipid also plays a major role in the pathways of this enzyme. Indeed, future investigation and a clear focus on this enzyme will be a great favor for understanding more about the exact mechanisms.
ACKNOWLEDGMENTS : We thank our supervisor for her part and management of our research.
CONFLICT OF INTEREST : None
FINANCIAL SUPPORT : None
ETHICS STATEMENT : None
References
- Zou K, Hinkley JM, Park S, Zheng D, Jones TE, Pories WJ, et al. Altered tricarboxylic acid cycle flux in primary myotubes from severely obese humans. Int J Obes. 2019;43(4):895-905.
- Fokin A. Effects of low citrate synthase activity, myostatin dysfunction, and caloric restriction on energy metabolism and body composition in mice (Doctoral dissertation, Lietuvos sporto universitetas). 2021.
- Martínez-Reyes I, Chandel NS. Mitochondrial TCA cycle metabolites control physiology and disease. Nat Commun. 2020;11(1):102.
- López M. Hypothalamic AMPK and energy balance. Eur J Clin Invest. 2018;48(9):e12996.
- Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505(7483):335-43.
- Dadsena S, King LE, García-Sáez AJ. Apoptosis regulation at the mitochondria membrane level. Biochim Biophys Acta (BBA)-Biomembr. 2021;1863(12):183716.
- Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288-98.
- Blüher M. Metabolically healthy obesity. Endocr Rev. 2020;41(3):bnaa004.
- Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020;126(11):1477-500.
- Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults: a meta-analysis of prospective studies. Sleep Med. 2014;15(12):1456-62. doi:10.1016/j.sleep.2014.07.018
- Milaneschi Y, Simmons WK, van Rossum EF, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. 2019;24(1):18-33.
- Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43(1):1-23. doi:10.1016/j.ecl.2013.09.009
- Shetty SS, Kumari S. Fatty acids and their role in type‑2 diabetes. Exp Ther Med. 2021;22(1):1-6.
- Batista TM, Haider N, Kahn CR. Defining the underlying defect in insulin action in type 2 diabetes. Diabetologia. 2021;64:994-1006.
- Lennicke C, Cochemé HM. Redox regulation of the insulin signalling pathway. Redox Biol. 2021;42:101964.
- Tobore TO. Towards a comprehensive theory of obesity and a healthy diet: The causal role of oxidative stress in food addiction and obesity. Behav Brain Res. 2020;384:112560.
- Čolak E, Pap D. The role of oxidative stress in the development of obesity and obesity-related metabolic disorders. J Med Biochem. 2021;40(1):1.
- Lopez-Yañez Blanco A, Díaz-López KM, Vilchis-Gil J, Diaz-Garcia H, Gomez-Lopez J, Medina-Bravo P, et al. Diet and maternal obesity are associated with increased oxidative stress in newborns: A cross-sectional study. Nutrients. 2022;14(4):746.
- Gonzalez A, Simon F, Achiardi O, Vilos C, Cabrera D, Cabello-Verrugio C. The critical role of oxidative stress in sarcopenic obesity. Oxid Med Cell Longev. 2021;2021.
- Leisegang K. Oxidative stress in men with obesity, metabolic syndrome and type 2 diabetes mellitus: Mechanisms and management of reproductive dysfunction. InOxidative Stress and Toxicity in Reproductive Biology and Medicine: A Comprehensive Update on Male Infertility-Volume One 2022 Jun 1 (pp. 237-256). Cham: Springer International Publishing.
- Razak HA, Lee LL, Sainuddin N, Hamzah R, Zainol NF, Mihat O. Exercise and obesity: activation of antioxidative pathway against oxidative stress. Int J Med Sci. 2021;6(1):54-64.
- Feng X, Zhang L, Xu S, Shen AZ. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: An updated review. Prog Lipid Res. 2020;77:101006.
- Jha V, Galati S, Volpi V, Ciccone L, Minutolo F, Rizzolio F, et al. Discovery of a new ATP-citrate lyase (ACLY) inhibitor identified by a pharmacophore-based virtual screening study. J Biomol Struct Dyn. 2021;39(11):3996-4004.
- Granchi C. ATP-citrate lyase (ACLY) inhibitors as therapeutic agents: a patenting perspective. Expert Opin Ther Pat. 2022;32(7):731-42.