Olive Leaves Extract Alleviate Diabetic Nephropathy in Diabetic Male Rats: Impact on Oxidative Stress and Protein Glycation
Hala A. H. Khattab 1,2*, Said S.Moselhy3, Alaa A.O. Aljafri1
1Food and Nutrition Department, Faculty of Home Economics, King Abdulaziz University, Jeddah, KSA.
2Nutrition and Food Science Department, Faculty of Home Economics, Helwan University, Egypt.
3 Biochemistry Department, Faculty of Sciences, King Abdul-Aziz University, Jeddah, KSA.
Email: haya_khattab @ hotmail.com
ABSTRACT
Diabetes (DM) is one of the top five causes of death worldwide. Controlling glucose level is vital for protecting the complications and improving the diabetics’ health. Olive (Olea europaea L.) leave extract (OLE) contains biologically active antioxidant phenolic compounds. This research was carried out to evaluate the effect of OLE on oxidative status and advanced glycation end products (AGEs) in DM rats. Male Wister rats (n=40, 200 ± 20 g) were divided into Group (1); Control rats and Groups (2-5); DM rats were intraperitoneal(i.p.) injected with STZ (65 mg/kg), only DM rats (fasting blood glucose >250 mg/dl) were randomly classified into DM , DM+ metformin (MT) (600 mg/kg) as reference drug, DM+OLE (200 mg/kg), and DM+OLE (400 mg/kg) groups. At the end of the experiment (6 weeks), blood and kidney samples were collected for biochemical and histopathological studies. Serum glucose, renal functions (creatinine (Cr), and blood urea nitrogen (BUN)), electrolyte ions (sodium (Na+) and potassium (K+)), as well as renal oxidative statusbiomarkers (nitric oxide (NO), malondialdehyde (MDA), superoxide dismutase (SOD), and AGEs) were determined. The results revealed that there were significant increases in glucose, Cr, BUN, and K+ with a significant decrease in Na+ levels, as well as significant decrease in renal oxidative stress and elevated AGEs levels compared to the control rats. Although MT treatment was more effective than OLE (400 mg/kg) in reducing glucose level, while OLE treatment was more effective than MT in reducing oxidative stress and AGEs levels. Oral administration of OLE (400 mg/kg) showed significant hypoglycemic and antioxidant effects as well as improved renal functions and inhibited AGEs levels compared to the DM rats. Also overcome most of the renal histopathological changes induced by DM. Therefore, co-administration of MT and OLE is recommended in preventing DM complications.
Keywords: Diabetes; nephropathy; rats; antioxidant; advanced glycation end products; olive leaves extract.
INTRODUCTION
Diabetes mallets (DM) is a chronic serious complex disease, that occurs either through deficiency of insulin production or not effectively insulin action. It is one of the greatest public health challenges for the 21st century [1, 2]. It is a major factor in several ailments including heart and kidney failure, stroke, loss vision, leg amputation, and others [3, 4]. Therefore, early interventions for pre-diabetic and diabetic patients by controlling the blood glucose through the combination of diet, physical activity, and drugs (if necessary); control blood lipids and blood pressure, and regular screening for damage to the kidney, eyes, and feet is important to facilitate the early treatment [2].
Phytotherapeutic cure prolong used in the management of several ailments, including olive (Olea europaea) leaves [3]. In the latest years, the extracts of several plants have been evaluated for their hypoglycemic and antioxidant activities. Olive leaves extract (OLE) has antioxidant and hypoglycemic properties mainly due to its phenolic compounds [4, 5]. It contains several antioxidants components as oleuropein, oleuropein aglycone, 3,4-dihydroxy phenyl ethanol, tyrosol, and verbascoside [6, 7]. Therefore, this research aimed to demonstrate the action of OLE (two doses) on oxidative status and AGEs in DM rats compared with metformin (MT) as a reference drug.
MATERIAL AND METHODS
Drug and chemicals
Streptozotocin (in white powder form, molecular weight 265.22 g/mol) was purchased from Sigma, USA. Metformin (Merck Serono, Middle East) in the tablet form was obtained from King Abdulaziz University Hospital. All chemicals were purchased from Alfa Aesar Chemical Co, USA and Invitrogen company California, USA. Kits for determinations of glucose-TV040CE004, creatinine (Cr) -TV033CE004, blood urea nitrogen (BUN)-TV075CE005, sodium (Na+) -TV071CE002, and potassium (K+)-TV067CE002 were purchased from Centronic Chemicals Co, Germany. Enzyme-linked immunosorbent assay ELISA kits for the determination of nitric oxide (NO)-E0703Ra, malondialdehyde (MDA)-E0156Ra, superoxide dismutase (SOD)-E0168Ra and advanced glycation end products (AGEs)-E0606Ra were purchased from Bioassay Technology Laboratory, China. Olive leaves (Olea europaea L.) extract (OLE) in liquid form was purchased fromCOMVITA Ltd, New Zealand, each 5 ml extract contains 22 mg oleuropein.
Experiment design
Male Wister rats (n=40, 200 ± 20 g) were obtained from the Animal experimental unit of King Fahd Medical Research Center, KAU. After one week of acclimatization, rats were divided into 5 groups (8 rats in each group). Group (1): Contr; rats received a single dose of 0.1 mol/L citrate buffer i.p., Groups (2-5); DM rats were i.p. injected with STZ (65 mg/kg), which were prepared in 0.1 mol/L citrate buffer (pH 4.5), after fasting for 12 h [8], then supplied with 5% sucrose solution orally [9]. Only rats with blood glucose in the fasting state higher than 250 mg/dl after 3 days were considered as being diabetic. Diabetic rats were randomly classified into; DM, DM+ Metformin (MT) (600 mg/kg) [10], DM+OLE (200 mg/kg), and DM+OLE (400 mg/kg). The dose, 200 mg/kg body weight, was chosen based on the previous study by El-Amin et al. [11].
Blood collection
After 6 weeks of treatment, blood samples were withdrawn from retro-orbital plexus of each rat, anesthetized with diethyl ether. Blood samples were centrifuged at 3000 rpm for 15 min in order to separate serum, then they were kept at -20oC until biochemical analysis.
Biochemical analysis
Serum glucose [12] creatinine (Cr) [13], blood urea nitrogen (BUN) [14], sodium (Na+), and potassium (K+) [15] were determined using enzymatic colorimetric kits.
Preparation of homogenate renal tissues
Renal tissue ( 0.1 g) was ground in a homogenization buffer (0.05 M Tris-HCl, pH=7.9, 25% glycerol, 0.1 mm EDTA, and 0.32 M (NH4)2SO4) containing a protease inhibitor tablet (Roche, Germany). Then centrifugation was performed at 13000 rpm and 4oC for 5 min. All supernatants were stored at -80 o C till analysis.
Renal oxidative status and advanced glycation end products (AGEs)
Nitric oxide (NO), malondialdehyde (MDA), superoxide dismutase (SOD) and advanced glycation end products (AGEs) were determined by double-antibody sandwich enzyme-linked immunosorbent assay ELISA kits.
Histopathological examination
Renal tissues from each group were processed, stained with hematoxylin-eosin (H&E), then examined under a light microscope [16].
Statistical
All results were expressed as mean ± SE. Data were evaluated using SPSS version 24, P< 0.05 was considered significant.
RESULTS
Effect of OLE on serum glucose in DM rats
In the DM group there was significant hyperglycemia compared to the Contr rats. There was a significant decline in serum glucose level in DM + MT compared to the DM group (P<0.001). Oral administration of OLE (200 mg/kg) and OLE (400 mg/kg) to DM rats showed significant improvement in glucose level as compared with the DM group. Interestingly high dose of OLE (400 mg/kg) showed significant improvement (P<0.05) in serum glucose level compared to the low dose of OLE (200 mg/kg).
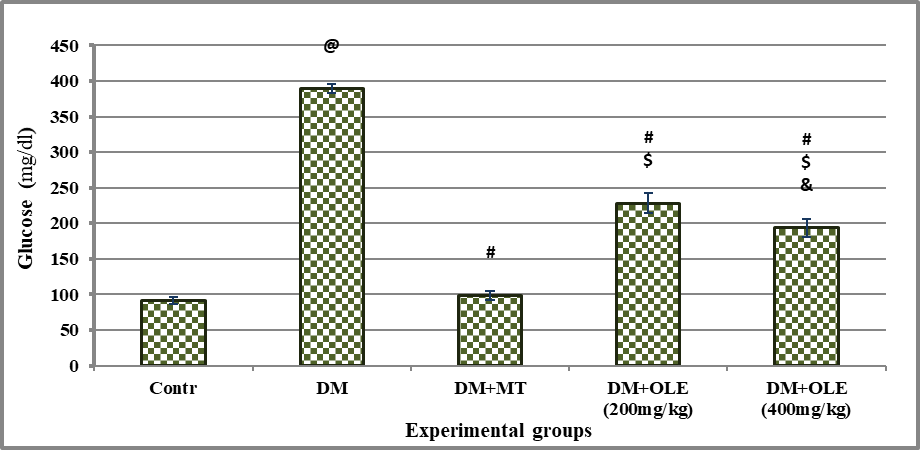
Significance was considered at p >o.o5. Results are presented as mean ± SE / 8 rats. @ Significant versus Contr group, # Significant versus DM group, $ Significant versus MT group, &Significant versus DM + OLE (200 mg/kg) group.
Figure 1: Effect of OLE on serum glucose level in DM rats
Effect of DM on renal functions and electrolyte ion biomarkers
There were significant increases in BUN, Cr, and ionic K+ (P<0.001) levels, with a significant decline in ionic Na+ level as compared with Contr rats with mean values [(17.51 ± 0.42 vs. 55.60 ± 1.33 mg/dl in BUN), (0.32 ± 0.01 vs. 0.68 ± 0.02 mg/dl in Cr), (4.75 ± 0.09 vs.5.81 ± 0.11 mmol/L in K+), and (149.31 ± 1.71vs.125.31 ± 2.76 mmol/L in Na+) for Contr vs. DM, respectively]. Administration of MT or OLE to DM rats showed significant improvement in serum levels of renal functions and ionic electrolyte, there were significant differences between the treated DM groups and the DM group. A dose-response improvement was observed in DM treated groups with OLE, where it showed significant difference (P <0.01) in renal functions and ionic electrolyte levels between DM+OLE (400 mg/kg) and DM+OLE (200 mg/kg) (Tables 1 & 2).
Table 1 : Effect of OLE on serum renal functions(BUN and Cr) in DM rats
|
Experimental groups |
BUN (mg/dl) |
Cr (mg/dl) |
|
Contr |
17.51 ± 0.42 |
0.32 ± 0.01 |
|
DM |
55.60 ± 1.33 a |
0.68 ± 0.02a |
|
DM+MT |
38.65 ± 1.46 b |
0.42 ± 0.03 b |
|
DM+OLE (200 mg/kg) |
38.02 ± 0.52 b |
0.42 ± 0.02 b |
|
DM+OLE (400 mg/kg) |
33.46 ± 0.51 b d |
0.48 ± 0.03 b d |
Significance was considered at p >o.o5. Results are presented as mean ± SE / 8 rats. a Significant versus contr group, b Significant versus DM group, c Significant MT group, d Significant DM + OLE (200 mg/kg) group.
Table 2 : Effect of OLE on serum electrolyte ions (Na+ and K+) in DM rats
|
Experimental groups |
Na+(mmol/L) |
K+(mmol/L) |
|
Contr |
149.31 ± 1.71 |
4.75 ± 0.09 |
|
DM |
125.31 ± 2.76a |
5.81 ± 0.11 a |
|
DM+MT |
132.63 ± 1.35 b |
5.38 ± 0.06 b |
|
DM+OLE (200 mg/kg) |
138.88 ± 1.13 b c |
5.11± 0.11 b c |
|
DM+ OLE (400 mg/kg) |
144.50 ± 1.31 b c d |
4.85± 0.07 b c d |
Significance was considered at p >o.o5. Results are presented as mean ± SE / 8 rats. a Significant versus contr group, b Significant versus DM group, c Significant MT group, d Significant DM + OLE (200 mg/kg) group.
Effect of OLE on renal antioxidant biomarkers
There was significant oxidative stress in DM rats as evidenced by significant (P<0.001) decline on renal NO and SOD content with significant (P<0.001) elevate on renal MDA content compared to the Contr group. Administration of MT or OLE to DM rats showed significant improvement in renal antioxidant content, there were significant differences between untreated and treated DM groups. A dose-response improvement was observed in DM treated groups with OLE, where it showed a significant difference (P<0.01) in renal antioxidant status between DM+OLE (400 mg/kg) and DM+OLE (200 mg/kg) (Figures 2-4).
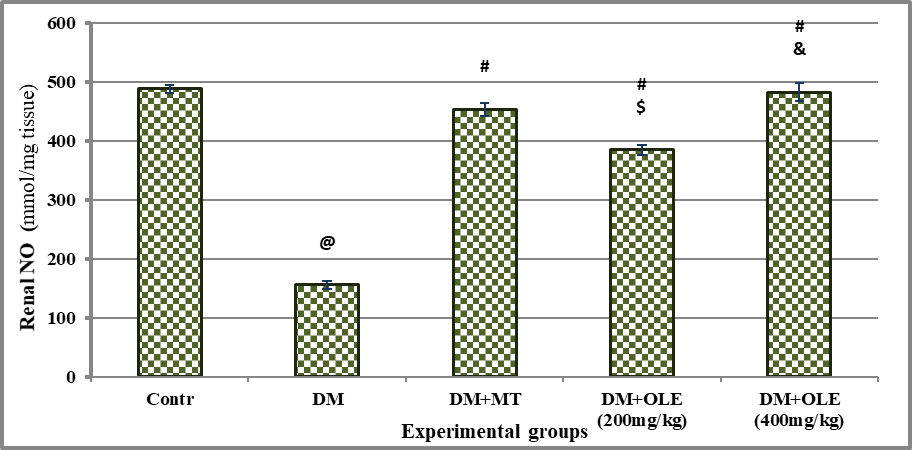
Significance was considered p > o.o5. Results are presented as mean ± SE / 8 rats. @ Significant versus Contr group, # Significant versus DM group, $ Significant versus MT group, &Significant versus DM + OLE (200 mg/kg) group.
Figure 2 : Effect of OLE on renal NO content in DM rats
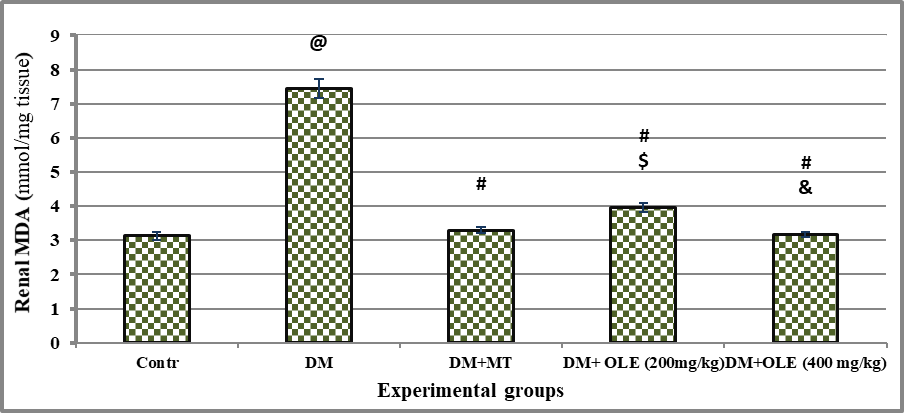
Significance was considered p > o.o5. Results are presented as mean ± SE / 8 rats. @ Significant versus Contr group, # Significant versus DM group, $ Significant versus MT group, &Significant versus DM + OLE (200 mg/kg) group.
Figure 3: Effect of OLE on renal MDA content in DM rats
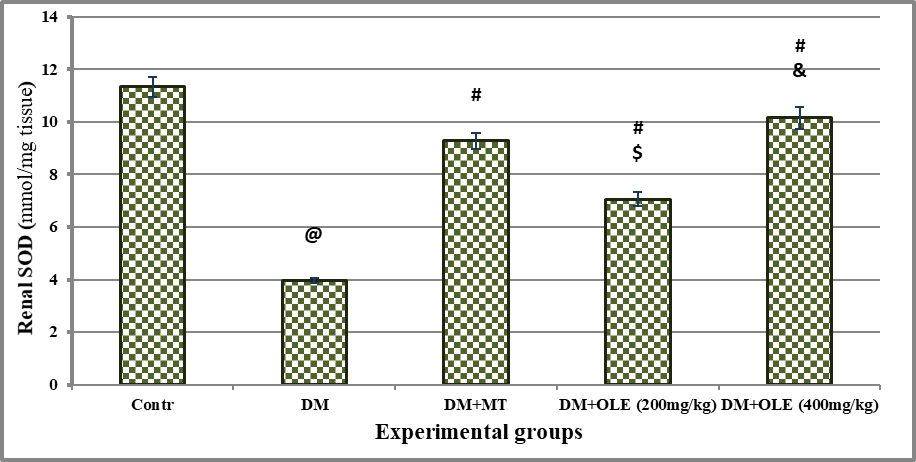
Significance was considered p > o.o5. Results are presented as mean ± SE / 8 rats. @ Significant versus Contr group, # Significant versus DM group, $ Significant versus MT group, &Significant versus DM + OLE (200 mg/kg) group.
Figure 4: Effect of OLE on renal SOD content in DM rats
Effect of OLE on advanced glycation end products (AGEs) in renal
The results revealed that the DM resulted in significant (P< 0.001) elevation in renal AGEs concentration compared to the Contr group with mean values of 123.23 ± 3.67 vs 627.38 ± 22.89 mmol/mg tissue in Contr and DM group, respectively. There was significant difference (P< 0.001) between the DM and all treated DM groups with MT, OLE (200 mg/kg) or OLE (400 mg/kg). Interestingly, there was significant difference (P< 0.01) on renal AGEs between DM+ MT and DM+ OLE (200 mg/kg). The improvement in renal AGEs was dose-dependent, where DM+ OLE (400mg/kg) showed a significant decrease in renal AGEs compared to DM+OLE (200mg/kg) group.
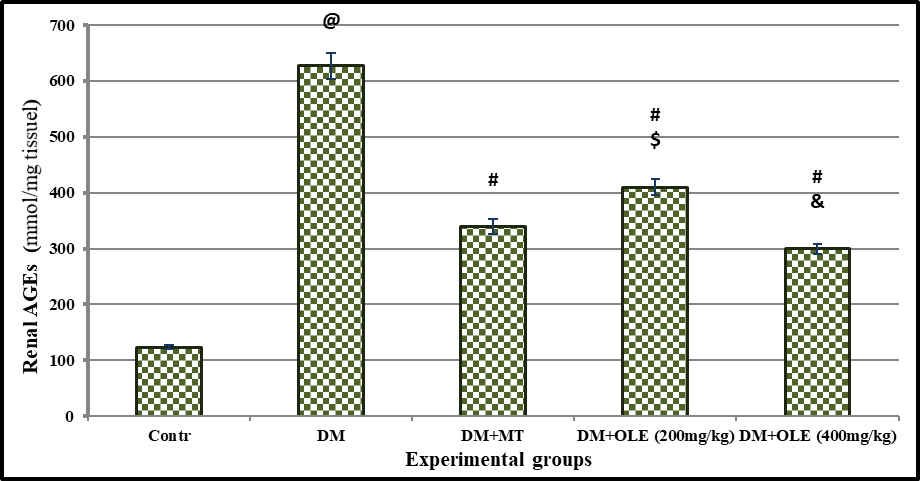
p> o.o5was considered significant. Results are presented as mean ± SE / 8 rats. @ Significant versus Contr group, # Significant versus DM group, $ Significant versus MT group, &Significant versus DM + OLE (200 mg/kg) group.
Figure 5: Effect of OLE on renal AGEs level in DM rats
Histopathological results
A Photomicrograph of the renal section from Contr rats showing the renal tubules and their normal epithelial lining in the medulla (Fig 6.A), the normal structure of the renal cortex, with narrow Bowman’s capsular space in the glomerulus. The proximal convoluted tubules have a narrow lumen and 4-5 cuboidal cell lining with an apical brush border and the distal convoluted tubule has a wide lumen and many cuboidal cell lining with apical nuclei (Fig 7.A). In the DM rats, renal section showed the sclerosed and contracted glomeruli, widespread of the dilated tubules with cloudy swelling of its epithelial lining (Fig 6.B). A large number of tubules showed the hydropic and the cloud swelling of the cytoplasmic degeneration of the tubular lining cells with dark pyknotic nuclei (Fig 7.B). In DM+MT, the renal section showed mild congestive changes in the glomeruli and renal tubules (Fig 6.C). Few glomeruli were with mild congestion and narrow Bowman’s capsule, while most of the tubules had a control appearance with normal vesicular nuclei (Fig 7.C). In DM+OLE (200 mg/kg), a renal section showed mild tubular degeneration (Fig 6.D). The tubules appear wide with atrophy of their epithelial lining and the hydropic degeneration of the tubular lining cells (Fig 7.D). While in DM+OLE (400 mg/kg), the renal section showed a marked decrease in the sclerosed glomeruli with a marked decrease in the intertubular congestion compared to the DM group (Fig 6.E). The higher magnification of a renal section showing glomeruli with a control appearance and a narrow Bowman’s capsule. The tubular cell lining showed normal vesicular nuclei in most of them together and focal dilated tubules with flat epithelial cell lining (Fig 7.E).
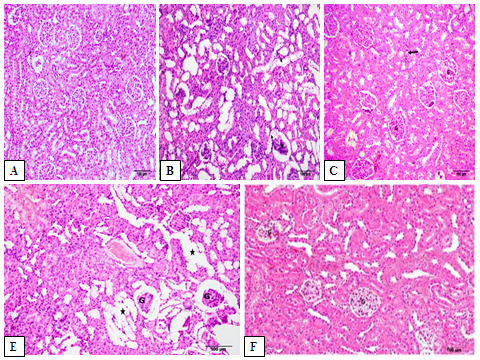
Figure 6: Photomicrograph showing the effect of OLE on renal histopathological changes in DM rats. Renal section from Contr rats showing the normal structure of the renal cortex [Fig. A]. In DM rats showing the sclerosed and contracted glomeruli (*). Notice the widespread of the dilated tubules with cloudy swelling of its epithelial lining (arrows) [Fig. B]. In DM+MT mild congestive changes in the glomeruli (G) and renal tubules. Notice the focal areas of intertubular congestion (thick arrow) [Fig. C]. In DM+OLE (200 mg/kg) rats showing slight tubular degeneration (*). Notice the sclerosed and contracted glomeruli (G) [Fig. E]. DM+OLE (400 mg/kg) showing a marked decrease in the sclerosed glomeruli (G). Notice the marked decrease in the intertubular congestion compared to the DM group [Fig. F].
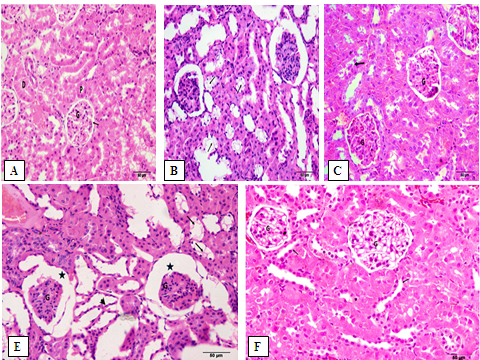
Figure 7: A higher magnification illustrating the effect of OLE on renal histopathological changes in DM rats. Renal section from Contr rats showing the normal structure of the renal cortex, and the glomerulus (G) with narrow Bowman’s capsular space (à). The proximal convoluted tubules (P) have a narrow lumen and 4-5 cuboidal cell lining with an apical brush border and the distal convoluted tubule (D) has a wide lumen and many cuboidal cells lining with apical nuclei [Fig. A]. In DM rats, a large number of the tubules show the hydropic and the cloud swelling of the cytoplasmic degeneration of the tubular lining cells (thin arrows) with dark pyknotic nuclei [Fig. B]. In DM+MT, few glomeruli (G) are with mild congestion and narrow Bowman’s capsule. Most of the tubules have a control appearance with normal vesicular nuclei [Fig. C]. In DM+OLE (200 mg/kg), the sclerosed glomeruli (G) is shown with wide Bowman’s capsule (*). The tubules appear wide with atrophy of its epithelial lining (arrowhead). Notice the hydropic degeneration of the tubular lining cells (arrows) [Fig. E]. In DM+OLE (400 mg/kg), glomeruli are with a control appearance and a narrow Bowman’s capsule (G). The tubular cell lining shows the normal vesicular nuclei in most of them together and focal dilated tubules with flat epithelial cell lining (arrows) [Fig. F].
DISCUSSION
Globally diabetesconsidersfrom the five causes of death [17]. Controlling the blood glucose level is vital for protecting complications and improving the diabetics’ health [18]. The available hypoglycemic drugs have numerous limitations, therefore herbal medicine can help to minimize diabetic complications. Olive (Olea europaea L.) leaves extract (OLE) contains biologically active phenolic compounds, which have antioxidant, hepatoprotection, anti-inflammatory, hypoglycemic, neuroprotection, hypolipidemic, and cardiovascular protection activities [19, 20]. Therefore, this research was conducted to assess the effect of OLE with two doses on the oxidative status and AGEs in DM rats compared to metformin (MT).
The results in this study revealed that there was a significant elevate on glucose level in the DM group compared to the contr group, which are in agreement with Prohp & Onoagbe [21], Zayed et al. [22], and Guex et al. [23]. These results could be explained by the inhibition of insulin secretion, β-cell destruction, and insulin resistance caused by STZ [24, 25]. Glucose level significantly decreased in DM+MT rats compared to the DM rats. This results in the same line as Horakova et al. [26] who showed that given MT orally induced decline in glucose level in DM rats. Moreover, Jin et al. [27] reported that metformin is potent to cure hyperglycemia, through glucose homeostasis regulation and diminished hepatic glucose production [28, 29]. The oral administration of two doses of OLE induced hypoglycemia; there was significant decrease in glucose concentration in DM + OLE groups compared to the the DM group. While as compared with MT drug, it was shown that there was significant difference between DM+MT and both groups treated with OLE (200 mg/kg) and (400 mg/kg). These results are in agreement with Abunab et al. [30], Abd El-Moneim et al. [31], and AlAttar & Alsalmi [32]. The hypoglycemic activity of OLE was demonstrated byareduction in starch digestion and absorption, inhibition of saliva and pancreatin α-amylases activity, increasing peripheral uptake of glucose, and potentiation of glucose-induced insulin release via major phenolic compounds of OLE, which have hypoglycemic and antioxidant activities in vitro and in vivo [33-36].
In the present study, the results indicated that there were significant elevation in BUN, Cr, and K+levels accompanied by significant reduction in Na+ level in the DM group compared to the Contr group. These results are in agreement with Jin et al. [27], Zhang et al. [37], and Jayaraman et al. [38]. This could be explained by metabolic disturbances via increasing xanthine oxidase activity [39]. Hyperglycemia caused movement of water out of the cells via increased osmolarity, thus subsequently induced dilutional hyponatremia (decreased serum sodium level) [40]. In the DM group, urine volume increases thus induces derangement in the balance homeostatic, which affects K+ concentration [41]. In this study, treatment DM with MT showed significant improvements in the kidney functions. Pan et al. [42] reported that the effect of metformin on the urine albumin/creatinine ratio are related to decreased blood glucose levels, blood pressure, and the degree of insulin resistance. Zhang et al. [37] reported that treatment with MT improved the majority of renal dysfunction parameters, indicating the preventive effects of MT in rats with T2D.
The administration of OLE to DM rats induced significant improvements in kidney function parameters. A dose-response trend was observed in DM treated groups with OLE, there were significant differences in serum BUN, Cr, K+, and Na+ levels between DM+OLE (400 mg/kg) and DM+OLE (200 mg/kg). These results are in the same line as Bader and Fouad [43], Karabag et al. [44], and Mohammed et al. [45]. The obtained data could be explained via OLE-induced regeneration of kidney glomeruli that improved the kidney filtration process [46]. Furthermore, the phenolic compounds and flavonoids in OLE that possess antioxidant and/or free radical scavenging activities, and revers histopathological changes in the kidney [47].
In the present study, the results revealed that DM resulted in significant reduction in renal content of NO and SOD, with significant elevation in renal levels of MDA and AGEs compared to the Contr group. The obtained data is in agreement with Palsamy and Subramanian [48], Chen et al. [49], Nasri et al. [50], Cai et al. [51], and Afify et al. [52]. Previous experimental and clinical studies reported that oxidative stress and impair endogenous antioxidant defense have a vital role in the development of DM complications. Thus explained via glucose auto-oxidation, glycated proteins, glycation of antioxidative enzymes, and stimulate cytochrome P450-like activity, which limits their capacity to detoxify oxygen radicals [53-58].
In the present study, treatment of DM rats with OLE (200 and 400 mg/kg) showed improvement in the antioxidant status in a dose-dependent manner, there were significant increases in renal levels of NO and SOD, with significant decreases in renal levels of MDA and AGEs compared to DM group. These results are in accordance with Hedeabet al. [59], Abd El-Rahman [60], Marta et al. [61], and Soliman et al. [62]. The antioxidant activity of OLE could be due to the bioactive compounds in OLE as flavonoids and flavones, which are superior hydroxyl radical scavengers and inhibit peroxidation [63]. Furthermore, polyphenol enriched OLE effectively, enhanced the SOD activity, and reduced the MDA in rats [64, 65].
In the present study, the renal sections of the DM rats showed sclerosed and contracted glomeruli, as well as widespread of the dilated tubules with cloudy swelling of its epithelial lining. A large number of tubules showed hydropic and cloud swelling of the cytoplasmic degeneration of the tubular lining cells with dark pyknotic nuclei. In DM rats treated with MT, the renal sections showed mild congestive changes in the glomeruli and renal tubules, as well as narrow Bowman’s capsule, and most of the tubules had a normal appearance with normal vesicular nuclei. These results are in agreement with Zhang et al. [37], Navpreet et al. [66], and Zayed et al. [22]. The obtained results could be explained by Forbes et al. [67] who reported that in DM hyperglycemia involved in the production of AGEs and free radicals, which induced cell death and renal dysfunction (diabetic nephropathy). In DM rats treated with OLE (400 mg/kg) the renal section showed nearnormal appearance with slight intertubular congestion and a narrow Bowman’s capsule. These findings are in agreement with Hedeab et al. [59]. The improvement of renal tissue could be explained by the antioxidant components in OLE, which reduce and prevent the complications related to oxidative stress, vascular health, inflammation, and endothelial function in DM [68].
CONCLUSION
The results of this study concluded that olive leave extract (OLE) may exert a protective role against STZ-induced diabetic nephropathy via an antioxidant mechanism.
ACKNOWLEDGMENTS
The authors acknowledge with thanks and appreciation the King Abdulaziz City for Science and Technology for their technical and financial support to the project of the research number (1-17-00-009-0036).
REFERENCES
- Cho, N.H., Shaw, J., Karuranga, S. , Huang, Y., da Rocha,f., Joao,D., Ohlrogge, A. and Malanda,B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract,2018 ; 138:271– 81
- WHO; World Health Organization. Diabetes country profile Saudi Arabia 2016, Geneva: Switzerland
- Eidi, A., Eidi, M. and Darzi, R. Antidiabetic effect of Olea europaea L. in normal and diabetic rats. Phytotherapy Research,2009 ; 23(3): 347–50.
- Kontogianni,V.G. and Gerothanassis,I.P. Phenolic compounds and antioxidant activity of olive leaf extracts. Natural Prod Res,2012; 26(2):186–9
- Salah, M. Abdelmele,H. and Abderraba,M.Study of phenolic composition and biological activities assessment of olive leaves from different varieties grown in Tunisia. Med Chem,2012; 2(5):107–11
- Jemai, H., Bouaziz, M., Fki, I., El Feki, A. and Sayadi, S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem Biol Inter, 2008 ;176 (2-3): 88–98.
- de Bock, M., Derraik, J. G., Brennan, C. M., Biggs, J. B., Morgan, P. E., Hodgkinson, S. C. and Cutfield, W. S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebocontrolled, crossover trial. PLOS ONE,2013 ; 8(3): 57622.
- Lutz,W. and Pardridge,M. Insulin therapy normalizes glucose transporter mRNA but not immunoreactivity transporter protein in streptozotocin diabetic rats. Metabolism,1993 ; 42(8):939–44.
- Peschke,E., Ebelt,H., Bromme,H.J.and Peschke,D. Classical and new diabetogens: Comparison of their effects on isolated rat pancreatic islets in vitro. Cell Molecul Life Sci,2000 ; 57(1):158–64.
- Derkach, K., Zakharova, I., Zorina, I., Bakhtyukov, A., Romanova, I., Bayunova, L. and Shpakov, A. The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect. PLOS ONE, 2019 ;14(3): e0213779. doi: 1371/journal.pone.
- El-Amin,M., Virk,P.,Elobeid,M.A.,Almarhoon,Z.M., Hassan,Z.K., Omer,S.A.,Merghani,N.M., Daghestani, M.H. and Al-Olayan, E.M. Anti-diabetic effect of Murraya koenigii (L) and Olea europaea (L) leaf extracts on streptozotocin induced diabetic rats, Pakistan J Pharmaceut Sci, 2013 ;26(2):359–65.
- Trinder, P. Determination of blood glucose using 4-Aminophenazone. J Clin Pathol,1969 ; 22(2):246.
- Henry, T.J. Clinical Chemistry Principles and Techniques, 1974 ; 2nd edi.,USA.
- Tietz,N.W. Textbook of Clinical Chemistry, Philadelphia. 1986.
- Berry, M.N., Mazzachi, R.D., Pejakovic, M. and Peake, M.J. Enzymatic determination of sodium in serum. Clin Chem, 1988 ;34(11):2295–8.
- Bancroft,J.D. and Cook,H.C.Manual of histological techniques.1998 ;Churchill Livingstone, New York:243.
- Go, H. K., Rahman, M. M., Kim, G. B., Na, C. S., Song, C. H., Kim, J.S., Kim, S. J. and Kang, H. S. Antidiabetic effects of yam (dioscorea batatas) and its active constituent, allantoin, in a rat model of streptozotocin-induced diabetes. Nutri,2015; 7(10): 8532–44.
- Ceriello, A. Postprandial hyperglycemia and diabetes complications: is it time to treat?. Diabetes, 2005; 54(1): 1–7.
- Ryan, D., Antolovich, M., Prenzler, P., Robards, K. and Lavee, S. Biotransformations of phenolic compounds in Olea europaea L. Scientia Horticulturae,2002 ; 92(2):147–76.
- Bulotta, S., Celano, M., Lepore, S. M., Montalcini, T., Pujia, A. and Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases. J of Transl Med, 2014 ;12: 219.doi: 10.1186/s12967-014-0219-9.
- Prohp, T. P. and Onoagbe, I. O. Plasma electrolyte concentrations in normal and streptozotocin-induced diabetic rats treated with extracts of Triplochiton scleroxylon K. Schum. Am J Res Comm, 2014 ; 2(5): 154–74
- Zayed,A.E., Saleh,A., Gomaa,A.M., Abd-Elkareem,M., Anwar,M.M., Hassanein,K.M., Elsherbiny,M.M. and Kotb,A.M.Protective effect of ginkgo biloba and magnetized water on nephropathy in induced type 2 diabetes in rat. Oxid Med and Cell Longev, 2018: 1094650. doi: 10.1155/2018/1094650.
- Guex,C.G.,Reginatoa,F.Z., de Jesusa,P.R., Brondanib,J.C., Hübscher Lopesc,G.H. and Bauermanna,L.D. Antidiabetic effects of Olea europaea L. leaves in diabetic rats induced by high-fat diet and low-dose streptozotocin . J Ethnopharmacol, 2019 ; 235 : 1–7. doi: 10.1016/j.jep.2019.02.001.
- Lenzen, S. The mechanisms of alloxan and streptozotocin, induced diabetes. Diabetologia,2008 ; 51(2):216–26.
- Kusakabe, T., Tanioka, H., Ebihara, K., Hirata, M., Miyamoto, L., Miyanaga, F., Hige, H., Aotani, D., Fujisawa, T., Masuzaki, H., Hosoda, K. and Nakao, K. Beneficial effects of leptin on glycaemic and lipid control in a mouse model of type 2 diabetes with increased adiposity induced by streptozotocin and a high fat diet. Diabetologia, 2009 ; 52(4): 675–83
- Horakova,A., Kroupova,P., Bardova,K., Buresova,J., Janovska,P., Kopecky,J. and Rossmei,M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Scientific Reports,2019 ; 9(1): 6156.
- Jin,J., Lim,S.W., Jin,L., Yu,J.H., Kim,H.S., Chung,B.H. and Yang,C.W. Effects of metformin on hyperglycemia in an experimental model of tacrolimus- and sirolimus-induced diabetic rats. Korean J of Internal Med,2017 ; 32 (2) :314–22.
- Galuska, D., Zierath, J., Thorne, A., Sonnenfeld, T. and Wallberg-Henriksson, H.Metformin increases insulin-stimulated glucose transport in insulin-resistant human skeletal muscle. Diabetes Metab,1991;17(1-2): 159–63.
- Madiraju, A. K., Erion, D.M., Rahimi, Y., Zhang, X.M., Braddock, D.T., Albright, R.A., Prigaro, B.J., Wood, J.L., Bhanot, S., MacDonald, M.J., Jurczak, M.J., Camporez, J.P., Lee, H.Y., Cline, G.W., Samuel, V.T., Kibbey, R.G. and Shulman, G.I. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature, 2014;510(7506):542–6 .
- Abunab, H., Dator, W.L. and Hawamdeh, S. Effect of olive leaf extract on glucose levels in diabetes-induced rats: A systematic review and meta-analysis. Diabetes, 2017;9(10):947–57.
- Abd El-Moneim,M.R., El-Beltagi,H.S., Sayed, A. and El-Ansary,A.E.Beneficial and potent effect of olive leaves extract on hyperglycemic state, kidney and liver function in STZ-induced type 2 diabetes mellitus. Fresenius Environment Bull,2018; 27(5): 3733–9.
- AlAttar.A.M. and Fawziah, A.A. Effect of Olea europaea leaves extract on streptozotocin induced diabetes in male albino rats. Saudi J of Biological Sci,2019; 24(1): 15–22.
- Gonzalez, M., Zarzuelo, A., Gamez, M.J., Utrilla, M.P., Jimenez. J. and Osuna, I. Hypoglycemic activity of olive leaf. Planta Medica,1992;58(6):513–5.
- Hamden, K., Allouche, N., Damak, M. and Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem Biol Interact,2009; 180(3):421–32.
- Wainstein, J., Ganz, T., Boaz, M., Bar Dayan, Y., Dolev, E., Kerem, Z. and Madar, Z.Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J Med Food,2012;15(7):605–10.
- Laaboudi,W. Eco-extraction of phenolic compounds from Moroccan olive fruits and leaves and their potential use as antimicrobial agents. European J Scientific Res,2015;132(3):255–65.
- Zhang, S., Xu, H., Yu, X., Wu, Y., and Sui, D. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Experimental and Therapeutic Medicine,2017; 14, 383–90. doi.org/10.3892/etm.2017.4475
- Jayaraman, R., Subramani, S., SheikAbdullah, S.H. and Udaiyar, M.Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed and Pharmacother, 2018; 97:98–106. doi: 10.1016/j.biopha.2017.
- Khanra, R., Bhattacharjee, N., Dua, T.K., Nandy, A., Saha, AJ. Kalita, J., Manna, P. and Dewanjee, S. Taraxerol, a pentacyclic triterpenoid, from Abroma augusta leaf attenuates diabetic nephropathy in type 2 diabetic rats. Biomed and Pharmacother,2017; 94: 726–41. doi: 10.1016/j.biopha.2017.07.112.
- Palmer, B.F. and Clegg, D.J. Electrolyte and acid base disturbances in patients with diabetes mellitus. New England J Med, 2015; 373(6): 548–59.
- Carlson, G.P. Fluids, electrolytes and acid-base balance. In Clinical Biochemistry of Domestic Animals.1997; Academic press, 5th edition, San Diego.pp. 486–513.
- Pan, Q., Xu, Y., Yang, N., Gao, X., Liu, J., Yang, W. and Wang, G. Comparison of acarbose and metformin on albumin excretion in patients with newly diagnosed type 2 diabetes: A randomized controlled trial. Med, 2016;95(14): e3247.
- Badr,A and Fouad,D Anti-apoptotic and anti-inflammatory effects of olive leaf extract against cisplatin-induced nephrotoxicity in male rats. Inter J Pharmacol,2016; 12(7): 675–88.
- Karabag, Ç. F., Hazman, O., Bozkurt, M. and Ince, S. Antioxidant status and anti-inflammatory effects of oleuropein in streptozotocin-induced diabetic nephropathy in rats. European J of Med Plants,2017;18 (2):1–10.
- Mohammed,H.A., Okail,H.A., Ibrahim,M.A. and Emam,N.M. Influences of olive leaf extract in the kidney of diabetic pregnant mice and their offspring. J Basic and Appl Zool,2018; 79(1):1–3.
- Eid, F. A., Shoman, H. H., Abu Elnaga, N.A. and Abed El-Halim, H. Effect of olive leaf extract on the kidney of pregnant diabetic rats and their fetuses. Inter J Advan Res, 2014;2(11):740–76.
- Helal, E. G., El-Wahab, S. M. A., El Refaey, H. and Mohammad, A. A. Antidiabetic and antihyperlipidemic effect of Balanites aegyptiaca seeds (aqueous extract) on diabetic rats. Egypt J Hospital Med, 2013;52(1): 725–39.
- Palsamy, P. and Subramanian, S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochimica et Biophysica Acta,2011; 1812(7):719–31.
- Chen, F., Zhang, H.Q., Zhu, J., Liu,K.Y., Cheng, H., Li, G.L., Xu, S., Lv, W.H. and Xie, Z.G.Puerarin enhances superoxide dismutase activity and inhibits RAGE and VEGF expression in retinas of STZ-induced early diabetic rats. Asian Pacific J Tropical Med,2012; 5(11):891–6.
- Nasri,R., Abdelhedi,O., Jemil,I., Daoued,I., Hamden,K.,Kallel,C., Elfeki,A., Senhadji,M.L., Boualga,A., Nasri,M. and Chaabouni,M.K. Ameliorating effects of goby fish protein hydrolysates on high-fat-high-fructose diet-induced hyperglycemia, oxidative stress and deterioration of kidney function in rats. Chem Biol Interact,2015;24:71–80.
- Cai, Y., Zhang, X., Xu, X. and Yu, Y. Effects of puerarin on the retina and STAT3 expression in diabetic rats. Experiment Therapeut Med,2017; 14(6): 5480–4.
- Afify, A.M.R., El-Beltagi, H.S., Fayed, S.A. and El-Ansary. A.E. In vivo correlation of olive leaves extract on some oxidative stress markers in streptozotocin-induced diabetes mellitus in rats. Grasasy Aceites,2018; 69 (1): 243.
- Jain, S.K.Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem,1989; 264(35):21340–5.
- Wolff,S.P.Diabetes mellitus and free radicals: free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull,1993; 49(3):642–52.
- Giugliano, D., Ceriello, A. and Paolisso, G.Diabetes mellitus, hypertension and cardiovascular diseases: which role for oxidative stress?. Metabolism, 1995;44(3):363–8.
- Haskins,K., Bradley, B. and Powers, K. Oxidative stress in type 1 diabetes. Annals of New York Acad of Sci,2003;1005:43–54.
- Joseph,L.E, Ira, D.G., Betty, A.M. and Gerold, M.G.Are oxidative stress activated signaling pathways mediators of insulin resistance and cell dysfunction?. Diabetes,2003;52(1):1–8.
- Johansen, J.S., Harris, A.K., Rychly, D.J. and Ergul, A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol, 2005;29(4):5.
- Hedeab,M. G., Sati, L.M., Elnaggar, H.M., Elgatlawey, Z.O., Eltwab, A.A., Elsaghayer, W.A. and Ali,H. Ameliorating effect of olive leaf extract on cyclosporine-induced nephrotoxicity in rats. Iranian J of Kidney Dis,2015;9(5):361–8.
- Abd El-Rahman,H.M. The effect of olive leaf extract and α-tocopherol on nephroprotective activity in rats. J of Nutr and Food Sci, 2016;6 (2): 1000479.
- Marta, N., Francisco,J.M. and Sonia,R.Olive leaf extract concentrated in hydroxytyrosol attenuates protein carbonylation and the formation of advanced glycation end products in a hepatic cell line (HepG2). Food Funct,2017; 8(3) : 944 –53.
- Soliman,G.A., Saeedan,A.S., Abdel-Rahman,R.F., Ogaly,H.A., Abd-Elsalam, R.M. and Abdel-Kader,M.S. Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats, molecular and biochemical study. Saudi Pharmaceut J, 2019;27(3):326–40.
- Arora, A., Nair, M. G. and Strasburg, G. M. Structure activity relationship for antioxidant activities of flavonoids in liposomal system. Free Radic Biol Med,1998; 24(9):1355–
- Cui, G., Qin, X., Zhang, Y., Gong, Z., Ge, B. and Zang, Y.Q. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J of Biol Chem,2009; 284(41):28420–9.
- Mansouri, E., Khorsandi, L. and Moaiedi, M.Z. Grape seed proanthocyanidin extract improved some of biochemical parameters and antioxidant disturbances of red blood cells in diabetic rats. Iranian J Pharmaceut Res, 2015; 14 (1): 329–34.
- Navpreet, K., Lalit,K. and Randhir,S. Dillenia indica L. attenuates diabetic nephropathy via inhibition of advanced glycation end products accumulation in STZ-nicotinamide induced diabetic rats. J Tradit and Complement Med, 2017; 8(1); 226–38
- Forbes, J.M., Coughlan,M.T. and Cooper, M.E. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes,2008; 57 (8):1446–54.
- Ahmadvand, H., Shahsavari, G., Tavafi, M., Bagheri, S., Moradkhani, M. R., Kkorramabadi, R. M., Khosravi, P., Jafari, M., Zahabi, K., Eftekhar, R., Soleimaninejad,M. and Moghadam, S. Protective effects of oleuropein against renal injury oxidative damage in alloxan-induced diabetic rats; a histological and biochemical study. J Nephropathol,2017; 6(3):204–9.
