Pharmacological Basis of Thymoquinone as a Putative Adjuvant Anticonvulsant – A Systematic Review
Lateef Mohiuddin Khan, Shahid Karim*
Department of Clinical Pharmacology, Faculty of Medicine, King Abdulaziz University, Saudi Arabia.
* Email: shahid.karim @ yahoo.co.in
ABSTRACT
Background: Nigella sativa (NS) has antioxidant and neuroprotective effects. Its concurrent use with AEDs could be a promising health strategy to prevent the damaging effect on neuronal cells during the episodes of seizures in addition to enhancing therapeutic effects and diminishing the adverse drug reactions of AEDs. Purpose: To provide the pragmatic perception of utilizing TQ as an adjuvant in antiepileptic therapies to potentiate their actions. Methods: The study utilizes systematic reviews on publications of previous studies obtained from scholarly journal databases including PubMed, Medline, Ebsco Host, Google Scholar, and Cochrane. The study utilizes secondary information obtained from health organizations using filters and keywords to sustain information relevancy. The use of search keywords and filters limits the study to relevant peer-reviewed journals. The study utilizes information retrieved from in vivo, in vitro and clinical studies captured in the peer-reviewed journals on “thymoquinone and epilepsy”, “thymoquinone and neuroprotection” “Nigella Sativa and epilepsy, “thymoquinone and AEDs” “model of epilepsy and thymoquinone”. Results: TQ was demonstrated to inhibit apoptosis and neuronal degeneration in the cerebral cortex. Furthermore, Nigella sativa oil and its active ingredient TQ protects brain tissue against radiation-induced nitrosative stress, TQ plays a crucial protective activity in the rat hippocampus and cortical neurons against Aββ1-42 and thus, it may be a promising agent for the treatment of Alzheimer’s disease. An interesting series of studies also reported that TQ has shown antiepileptic effects. Akhondian et al. reported that orally administered TQ reduces intractable pediatric seizures. Also, Hosseinzadeh et al., showed that TQ administered intracerebroventricularly, for epileptiform activity induced by using pentylenetetrazole (PTZ) in rats, prolonged the latency to first seizure, and decreased seizure count and the periods of tonic-clonic seizure in a dose-dependent manner. In another study, orally administered TQ prolonged the first seizure latency, decreased seizure count, and eliminated lethality in PTZ-induced epilepsy. TQ has a protective and inhibitory effect on a penicillin epilepsy model, as with the other experimental epilepsy models. Conclusion: The current approach to AED discovery is effective for identifying drugs that are useful for the symptomatic treatment of seizures. However, such an approach cannot be adequate to develop therapies for preventing and modifying the development of epilepsy in a susceptible person. Undoubtedly, TQ is demonstrated as an ideal adjuvant to antiepileptic therapies by potentiating their actions and retreating their adverse effects.
Key words: Thymoquinone, Epilepsy, GABA, Nigella Sativa, Neuroprotection.
INTRODUCTION
Epilepsy is a common and heterogeneous neurological disorder arising from biochemical and molecular events that are incompletely understood. Pharmacotherapy of epilepsy comprises 24 different anti-epileptic drugs, (AEDs) yet the exact anticonvulsant mechanisms are not fully elucidated for most of them [1–3]. Initial work of Merrit and Putnam in the 1940s commenced with the discovery of antiepileptic drugs based on screening candidate drugs in predictive animal models without regard to mechanisms of drug action [4, 5].
Remarkably, AEDs often employed in clinical practice with the modest perception of their pharmacodynamic effects, their symptomatic benefit in the clinical practice is subsequently followed by an intensive exploration of potential molecular targets by which they principally act to confer protection against the seizures [6]. On the contrary, the last two decades revealed the buildup of numerous exclusively novel AEDs and their target, notably levetiracetam, gabapentin, pregabalin, and ezogabine (formerly, retigabine). This target-based drug discovery effort represents a new paradigm in antiepileptic drug discovery [7].
Despite this additional stream of innovative AEDs, adequate seizure control in several patients is still not accomplished.
This has led investigators to look for the approaches in which damage to the brain culminates in a chronic and long-lasting state of impulsive seizures, a process identified as epileptogenesis. Escalating shreds of evidence demonstrate that numerous epileptic episodes transpire as the phenomenon of epileptogenesis which comprises of the progressive configuration of fresh persistent excitatory circuits, for instance, mossy fiber sprouting [8, 9], the discriminatory and progressive loss of unambiguous and susceptible Gabaergic interneurons [10], whereas enhancement of glutaminergic activity becomes more obvious [11].
Furthermore, excitatory axons undergoing mossy fiber sprouting accompanied by the organization of novel synaptic connectivity is recognized as an omnipresent epileptogenic reaction to hippocampal or neocortical injury [12].
Accumulating shreds of evidence suggest that excitotoxic, necrotic, and programmed (apoptotic) cell deaths are being analyzed in brain tissue from identified human focal epilepsy cases. Moreover, the molecular, structural, and physiologic plasticity of neural circuits in both human epilepsy and experimental models also remains under intense scrutiny, seemingly this area perhaps continues to provide essential information on the mechanisms underlying reorganization and synaptogenesis [4].
It is noteworthy that reactive astrocytosis leads to the expression of neuronal N-methyl-D-aspartate (NMDA) receptors and the release of glutamate [13]. Despite the varied primary pathology of epileptic seizures, the mechanisms involved in generating and spreading epileptic discharges converge on a common cellular pathology in which the excitatory glutamatergic system plays a key role. Compelling neurophysiologic, pharmacologic, biochemical, and anatomical evidence has been accumulated over the last several decades firmly implicating ionotropic N-methyl-D-aspartate (NMDA), 2 and a-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA)/ kainate and metabotropic glutamate receptor-mediated mechanisms in epileptic seizures. Excitatory glutamatergic mechanisms are involved during both acute, transient, evoked transient, evoked and long-term [14].
On the other hand with keeping in mind that the release of excitatory neurotransmitters such as glutamate and aspartate has important roles in the brain tissue damage. Also, the rats which had experienced recurrent seizures showed damages to the parts of the brain such as the hippocampus and other parts of the limbic system. PTZ induced repeated seizures damaged to hippocampal neurons of the rats were significantly prevented by NS extract [15, 16].
In general, these findings provide novel perspectives in regards to using TQ as an adjuvant in antiepileptic therapies either to potentiate their actions, this may further worth investigating with contemporary AEDs. The basic essence of integration of TQ as adjuvants with AEDs is directed towards reducing their essential doses which in turn plays an eminent role to circumvent and diminish their inherent adverse effects with overall potentiation of their therapeutic activities. Given widely accepted insight of epilepsy that the epilepsy is frequently associated with oxidative stress [17], it becomes plausible to utilize safely-applicable anti-oxidants concomitant with AEDs to alleviate the overall burden of the disease and to minimize drug-related adverse effects.
Nigella Sativa
- Characteristics of the plant, seeds, and powder
Nigella sativa (N. sativa) is an annual plant commonly named as “black seed” or “black cumin”. It is in the family Ranunculaceae and is generally native to North Africa, Southern Europe, and Southwest Asia. Its cultivation is common in the Middle East (including Syria and Saudi Arabia), South Europe (Turkey), India, and Pakistan [18].
N. sativa can grow to 20-90 cm tall. The flowers include 5-10 petals and their common colors are yellow, white, and pink. The leaves are finely divided with narrow and linear leaf segments. The plant has a large fruit containing 3-7 united follicles with multiple seeds within each one [19, 20]. Plant seeds are small and angular with an outer black and inner white color. Their taste is bitter and the odor is slightly aromatic. The seeds are stored as planting material as well as a spice for one year with minimal exposure to the air to avoid loss of aroma [21]. Microscopically, epidermis layers are shown on the transverse sections of seeds, whereby the cells appear elliptical and thick-walled. The seed powder is brownish-black with oil globules and parenchymatous cells as seen under a microscope [19].
- Chemical composition of the seeds
The fixed oils, proteins, saponin, and alkaloids represent 36%-38% of the seed with 0.4%-2.5% essential oils. The predominant constituents of fixed oils are unsaturated fatty acids, such as eicosadienoic and arachidic acids [22]. For the essential oils, the main components are thymoquinone (TQ, 28%–57%, Figure A), carvacrol (6%–12%), ρ-cymene (7%–16%), t-anethole (0.25%–2.3%) and longifolene (1%–8%) [23]. In general, the seeds contain four alkaloids. An indazole nucleus is integrated into the chemical structure of nigellicine (Figure B) and nigellidine (Figure C), while the remaining alkaloids are regarded as isoquinolines (nigellimine and nigellimine N-oxide in Figure D and Figure E, respectively) [24–26]. Kumara and Huat (2001) have isolated an additional constituent N. sativa seeds (alpha-hederin), which expressed an anti-tumor activity [27].
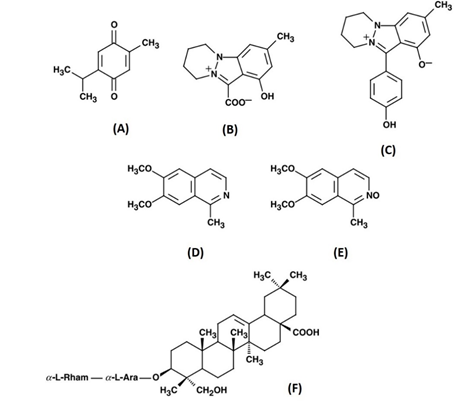
Figure 1: The chemical structure of some major constituents of N. sativa seeds. (A) thymoquinone; (B) nigellicine; (C) nigellidine; (D) nigellimine; (E) nigellimine N-oxide; (F) α-hederin. Adapted from Ali and Blunden [23]
N. sativa seeds contain 26.7% protein, 24.9% carbohydrates, 8.4% crude fiber, and 4.8% total ash. Minerals, such as copper, zinc, and iron are also abundant. There is a considerable amount of carotene, which could be converted into vitamin A [28]. The most abundant quinine constituent (TQ) produces dithymoquinone and high condensation products on storage. It is the major impactful substance for the pharmacological properties of black seeds.
- Therapeutic uses
Infectious diseases have always constituted the most serious health issue in the world, at least until the beginning of the 20th century when chronic degenerative diseases started to develop in developed countries [29]. Traditionally, N. sativa seeds and oils were used in the Middle East and Southeast Asia for multiple diseases and conditions, such as asthma, bronchitis, and rheumatic diseases. Seed-based tinctures were also utilized for the treatment of diarrhea, indigestion, loss of appetite, and worms. Topically, black seed oil was regarded as a local anesthetic and antiseptic [30–32].
Based on such traditional evidence, the biological activities of N. sativa have been extensively investigated, revealing a wide spectrum of therapeutic applications (Table ).
Table 1: Summary of the main therapeutic effects of N. sativa.
|
N. sativa Component |
Subjects |
Method of administration/quantification |
Therapeutic effect |
Reference |
|
Antibacterial Effects |
||||
|
Ground black seeds |
In-vitro investigation |
Modified paper disc diffusion |
Inhibition of S. aureus growth |
[33] |
|
Crude alkaloid and water extracts |
In-vitro investigation |
N/A |
Inhibition of selected G-ve bacteria |
[34] |
|
Ethanolic extract |
In-vitro investigation |
Disc diffusion and in-agar dilution methods |
MRSA strains were sensitive |
[35] |
|
Seeds |
Adult patients positive for H. pylori |
Oral |
Inhibited H. pylori activity |
[36] |
|
Antifungal |
||||
|
Aqueous extract |
BALB/C mice |
I.P |
Inhibit C. albicans |
[37] |
|
Ether extract |
In-vitro investigation |
Agar diffusion method |
Antidermatophyte activity |
[38] |
|
Anti-schistosomiasis |
||||
|
Oil |
Mice |
Oral |
Lowering the number of Schistosoma mansoni worms in the liver when compared to praziquantel |
[39] |
|
Seeds |
|
In-vitro incubation of crushed seeds with the different stages of S. mansoni |
Inhibition of all parasitic stages of S. mansoni |
[40] |
|
Anti-oxidant |
||||
|
Methanol extracts |
Cancer cell lines |
Oxygen radical absorbance capacity method and a cell-based assay |
Antioxidant |
[41] |
|
Seed powder |
Swiss Albino rats |
Oral |
Antioxidant |
[42] |
|
Seeds |
Broiler chicks |
Oral |
Prevent oxidative stress in the liver |
[43] |
|
Antidiabetic |
||||
|
Aqueous extract |
STZ-induced diabetic rats |
I.P injection |
Hypoglycemic |
[44] |
|
N. sativa extract and oil |
STZ-induced diabetic rats |
I.P injection |
Reduction of tissue MDA and serum glucose Increased tissue SOD and serum insulin |
[45] |
|
Volatile oil |
STZ-induced diabetic rats |
Oral |
Minimizing the morphological changes of pancreatic β-cells and preserving their integrity |
[46] |
|
Anti-inflammatory and anti-allergic |
||||
|
Oil in capsules |
Patients with allergic rhinitis |
Oral |
Reduce nasal congestion, sneezing, and runny nose |
[47] |
|
Immunomodulation |
||||
|
Aqueous extract |
In-vitro investigations |
[(3)H]-thymidine incorporation and Griess assay, |
Enhance splenocyte proliferation Suppress the secretion of IL-6, TNF-alpha, and NO by macrophages (anti-inflammatory) |
[48] |
|
Methanolic extract |
Balb/c mice |
I.P |
Enhance WBC count and increase bone marrow cellularity |
[49] |
MRSA: methicillin-resistant Staphylococcus aureus; G-ve: gram-negative; I.P intraperitoneal; MDA: pancreatic tissue malondialdehyde; SOD: superoxide dismutase; STZ: Streptozotocin; IL-6: Interleukin 6; TNF: tumor necrosis factor; NO: nitric oxide; WBC: white blood cells;
- The toxicological profile
Generally, black seeds have a low-level of toxicity. The hepatic and renal functions in rats were not affected following an intraperitoneal (IP) injection of N. sativa extract at high doses (50 mg/d) for 5 days [50]. Likewise, no toxic effects were reported in mice given fixed oils at a dose of 10 mL/kg for 3 months orally with normal histopathological findings [51]. In humans, the topical application of pure N. sativa oil caused allergic contact dermatitis in two cases [52].
Thymoquinone
The chemical structure of the monoterpene molecule, TQ, is 2-methyl-5-isopropyl-1, 4-benzoquinone (Figure A). TQ is regarded as the most bioactive constituent of the volatile oil of N. sativa. It was first extracted in 1963 from the essential oil by El–Dakhakhny [53]. In addition to N. sativa, other sources of TQ include some plants of the genera Lamiaceae [54, 55] (such as Coridothymus, Agastache, Monarda, and Origanum), Tetraclinis, Cupressaceae, and to a less extent in the seeds of Nigella arvensis [56]. TQ extraction from essential oil could be attained by supercritical fluid extraction. This can be performed using food-grade liquid carbon dioxide in high-pressure cylinders [57]. Hydro-distillation could be also performed using a Clevenger-type apparatus [58].
- Thymoquinone Pharmacokinetics
The pharmacokinetic properties of TQ have been investigated in animals following its
administration via IV, oral, intragastric, or IP routes, showing promising clinical and pharmaceutical applicability. The most commonly reported doses of TQ were 5 mg/kg
for intravenous injections and 20 mg/kg if given orally [59]. Pharmaceutical consultation is an integral part of the retail sale of drugs to the public [60, 61].
The mean calculated clearance was 7.19 ± 0.83 ml/kg/min and the volume of distribution at steady state was estimated to be 700.90±55.01 ml/kg after IV administration. Additionally, the estimated absorption half-life (t1/2) was approximately 217 min. and the elimination t1/2 was 63.43±10.69 min. for IV injection as well as 274.61±8.48 min. for oral administration. Protein binding of TQ has been reported to be as high as 99% [59]. In sum, these results indicate a slow absorption and quick elimination of TQ following oral administration.
- Pharmacological usages
TQ has several pharmacological potentials against a set of diseases. Reports have shown a cardioprotective effect of TQ against myocardial lesions that mimic myocardial infarction induced by isoproterenol in rats [62]. In rabbits fed cholesterol-rich diets, TQ administration at a dose of 20 mg/kg/d caused favorable effects on serum glucose and insulin as well as liver enzymes [63]. Nader et al. (2010) suggested a role of TQ in reducing low-density lipoprotein (LDL) levels via modulation of LDL-cholesterol uptake through a specific gene-targeted mechanism [64].
For the antibacterial activity, it has been suggested that TQ enhances the potency of verified antibiotics and antiseptics by 4 times [65]. Furthermore, Halawani (Halawani 2009) revealed a promising synergism of TQ with cephalexin, gentamicin, and tetracycline against S. aureus [66]. Also, TQ showed an antifungal action by inducing a marked inhibition of eight species of dermatophytes compared to griseofulvin and an anti-parasitic activity against Giardia lamblia and Entamoeba histolytica [62]. When co-administered with curcumin, TQ synergistically elevated antibody levels and promoted cytokine gene countenance against the H9N2 strain of avian influenza virus in Turkeys [67].
In vivo studies in rats have shown high efficacy of TQ in the treatment of acute gastric ulcer [68], renal ischemia/reperfusion injury [69], rheumatoid arthritis (via inhibition of tumor necrosis factor “TNF-α” and interleukins “IL-1β”) [70], streptozotocin (STZ)-induced diabetes [71], diabetic neuropathy [46], acute respiratory distress syndrome [72], and hepatic toxicity [73]. Besides, it reduced the progression of pulmonary fibrosis [74] and ameliorated gentamicin-induced renal failure [75].
- Neuroprotective effects of TQ
The neuroprotective effects of TQ have grabbed the attention of several researchers through enhancing the learning and memory aspects and preventing diabetes-induced cognitive impairment, possibly by reducing the oxidative stress [76]. Seemingly, TQ is the most effective neuroprotective constituent in N. sativa as it exhibited a 10-fold potency in inhibiting nonenzymatic peroxidation as compared to N. sativa oil [77]. Another neuroprotective mechanism was reported in ischemia-reperfusion injury of the spinal cord as evidenced by reducing the oxidative products (such as nitric oxide), enhancement of antioxidant enzymes (glutathione-peroxidase and superoxide dismutase (SOD), inhibition of motor neuronal apoptosis and decreasing the production of TNF-α and IL-1 [78].
For epilepsy, TQ was beneficial in alleviating seizures induced by either maximal electroshock (MES) or pentylenetetrazole (PTZ) when injected IP at doses of 40 and 80 mg/kg along with reducing mortality rates to 71.4% and 100% for both doses, respectively [79]. Such neuroprotective effect was apparent as the myoclonic seizures have been significantly prolonged and their durations were greatly reduced in this epileptic model that mimics that of petit mal. Given that PTZ causes a reduction of the GABAergic tone, it is plausible that TQ caused an increase in the GABAergic tone [79]. An assumption that should be taken into consideration is that TQ causes indirect activation of κ-opioid receptors [80]. Indeed, such activation has been linked with an anticonvulsant activity due to blockade of Ca2+ channels [81]. A relatively similar mechanism has been proposed by Ullah et al. (2015) when they treated rats having PTZ-induced generalized epilepsy with TQ (oral, 40 mg/kg) and vitamin C (IP, 250 mg/kg). Rats pre-treated with either therapies or a combination therapy showed a significant attenuation of seizure activity and decreased neurodegeneration and mortality. Furthermore, the onset of seizures was prolonged and high-grade seizures were diminished. Both treatments or either of them reversed multiple pathogenic factors that have been formerly enhanced during seizures, such as reduction of the expression of GABAB receptors, inhibition of the phosphorylation of cAMP response element-binding protein (CREB), and reduction of calcium/calmodulin dependent protein kinase II (CaMKII) pathway [82].
In another epileptic rat model, Shao and co-workers investigated the role of TQ in reducing brain injury in SE. Seizures were induced by lithium and pilocarpine injection and a Racine scale and an electroencephalogram were utilized to quantify seizure severity [83]. TQ was administered IP (10 mg/kg) in a rat group and another group 31 received normal saline as a control. The authors found that the total power of seizures, as well as seizure severity, was significantly lower in the TQ group. Consistent with the fact that SE would eventually lead to neuronal injury and death [84] via neurotoxicity, inflammation, and inducing oxidative stress, TQ offers protection through its potent antioxidant effects. This was confirmed by the increased expression of several antioxidants, including Nrf2 and heme oxygenase-1 (HO-1) proteins as well as SOD [83] in the TQ-injected rats when compared to the control group. Another earlier evidence of TQ effects on SE using the same seizure-induced model has suggested another protective mechanism, which suggests that TQ follows an anti-inflammatory pathway to reduce seizure activity by inhibiting cytokine expression (TNF-α and cyclooxygenase-2 (COX-2) and inhibiting the inflammatory-mediating NF-κB signaling [85]. TQ had also a significant effect on another type of intractable epilepsy, namely temporal lobe epilepsy (TLE), which is characterized by increased oxidative stress, neuronal loss, and increased astrogliosis [86–88]. Dariani and team have shown that TQ injection (10 mg/kg) in an intrahippocampal kainate rat model led to attenuation of multiple factors involved in the pathogenesis of increased seizure activity, including attenuation of malondialdehyde (MDA, an important indicator of oxidative stress) in the hippocampus, astrogliosis, hippocampal neuronal loss and mossy fiber sprouting intensity [89].
Consistent with the aforementioned findings, Beyazcicek et al. (2016) revealed a favorable impact of TQ in rats with penicillin-induced epilepsy. They utilized different doses of TQ (10, 50, and 100 mg/kg, IP) and compared their outcomes with other groups receiving a vehicle (dimethylsulfoxide), sham, or control. The results showed that TQ prolonged latency time and decreased the frequency and power of epileptic seizures as compared to the vehicle-receiving group [90]. However, the authors performed no additional tests to investigate the potential mechanisms involved in such antiepileptic effects.
Overall, the most acceptable mechanisms of action of TQ were summarized in Table 2.
Table 2: The supposed mechanisms of action of TQ in different epileptic models
|
Epileptic model |
Potential mechanism of action of TQ |
|
Petit mal |
κ-opioid receptor-mediated augmentation of GABAergic tone |
|
Status epilepticus |
Activation of an antioxidant mechanism via increasing the expression of Nrf2 and HO-1 proteins and SOD |
|
Status epilepticus |
Inhibition of NF-κB signaling which mediates inflammation |
|
Temporal lobe epilepsy |
Attenuation of malondialdehyde in the hippocampus, astrogliosis, hippocampal neuronal loss and mossy fiber sprouting intensity |
|
PTZ-induced generalized epilepsy |
Enhancing the expression of GABA B1 receptors and activation of CREB and CaMKII pathways |
CaMKII: calcium/calmodulin-dependent protein kinase II; CREB: cAMP response element-binding protein; GABA: γ-aminobutyric acid; HO-1: Heme oxygenase-1; NF: Nuclear Factor; SOD: Superoxide dismutase; TQ: Thymoquinone.
- TQ as an adjuvant to reduce drug toxicity
The experimental investigations have shown a promising role of TQ when administered with AEDs. Raza et al. (2006) evaluated the combined effect of TQ with sodium valproate (SVP) in PTZ- and MES-induced seizure models in rats to reduce the potentially toxic adverse effects of SVP given in the drinking water at a dose of 1300-1500mg/kg/day for 3 weeks [91]. Such toxic doses led to a marked elevation of liver enzymes and an increase in lipid peroxidation as well as the reduction of glutathione in hepatocytes [92]. A combination of TQ (5 mg/kg/d) and SVP caused normalization of hepatic enzymes and GSH recovery though the abnormal lipid peroxidation was not affected (Table 2). TQ caused a significant reduction of the median anticonvulsant effective doses (ED50) of SVP from 161 to 112 mg/kg against the PTZ-induced seizures and from 170.1 to 124.8 mg/kg in MES-induced epilepsy. Despite the lack of a potent impact on lipid peroxidation in the study of Raza et al. (2006), it seems that the hepatoprotective effect of TQ is initiated via an antioxidant mechanism, which has been indicated by an in-vitro inhibition of non-enzymatic lipid peroxidation in liver homogenate [75, 91]. Indeed, these results suggest a considerable hepatoprotective effect of TQ [93, 94] that could induce a beneficial interaction with SVP to help reduce its dose and eliminate the expected hepatotoxic and teratogenic implications [95].
Another experimental study has employed a kindling model, which has been identified as attaining a progressive form of generalized tonic-clonic seizures via repetitive application of electrical stimulation of the brain or repetitive administration of sub-convulsive doses of PTZ [96]. Mostafa et al. (2012) have investigated the effects of concomitant administration of TQ and PB in PTZ-kindling in rats. A subgroup of rats was divided into five groups receiving either PTZ injection alone or followed by PB (30 mg/kg, IP), TQ (20 mg/kg, oral), or a combination of both as well as a control group injected with normal saline. Although using either treatments alone or in combination was associated with a significant reduction of seizure activity, the addition of TQ was insignificant seizure control when compared to PB administration. However, the use of combined TQ and PB was associated with a marked improvement of antioxidant parameters like GSH levels, MDA levels, and the activity of GSH-peroxidase GSH-reductase as compared to the PB-receiving group (Table 3). As such, it is plausible that TQ exerted an antioxidant action that increased the efficacy of PB in the kindled rats [97].
Table 3: The potential effects of TQ to reduce the AED adverse effects.
|
Epileptic model |
Concomitant AED |
The potential mechanism |
Reference |
|
PTZ- and MES-induced seizures |
SVP |
Normalization of liver enzymes, glutathione recovery and potential inhibition of lipid peroxidation |
[91] |
|
PTZ-induced kindling |
PB |
Neuroprotective actions via an antioxidant mechanism through the significant improvement of GSH levels, MDA levels, and the activity of GSH-peroxidase GSH-reductase |
[97] |
AED: Antiepileptic drug; GSH: Glutathione; MDA: Malondialdehyde; MES: Maximal electroshock; PB: Phenobarbital; PTZ: Pentylenetetrazole; SVP: Sodium valproate; TQ: Thymoquinone.
Several studies have investigated the effects of TQ on epilepsy as an adjuvant to AEDs in humans. Akhondian et al. (2007) investigated the effects of the aqueous extract of black seed (40 mg/kg/8h) on refractory epilepsy in children in a crossover clinical trial. In this study, a group of water extract-receiving patients was compared to a placebo-receiving group concomitant with AEDs that have been administered for at least one-month prior allocation time. The authors found a significant reduction of seizure frequency in the group that received N. sativa water extract [98]. Using TQ exclusively, the same group subsequently conducted a double-blinded clinical trial on children having refractory epilepsy, where TQ was administered at a dose of 1 mg/kg (TQ was given orally as a syrup with a concentration of 25 mg/ml). Twenty-two patients were allocated to two groups, where they received either TQ or placebo for 4 weeks concomitant with the pre-existing AEDs, including clonazepam, CBZ, PHT, PB, lamotrigine, and valproate. After cross-over of treatments between placebo and TQ for an additional 4 weeks, a significant reduction of seizure frequency in the TQ group as compared to the placebo [99].
However, another prospective crossover pilot clinical study has shown relatively contrasting outcomes Shawki and co-workers used black seed oil to assess the concomitant effects of TQ with the pre-existing AEDs versus placebo 35 in children with intractable epilepsy (n=30). The used AEDs were CBZ, valproate, or a polytherapy. The authors found no significant effect of TQ on seizure activity as compared to placebo even when the interventions were reversed among the groups [100]. Nonetheless, the patients showed a significant improvement in oxidative stress markers as shown by the significant reduction of total antioxidant capacity levels in the treated groups when compared to healthy controls (n=5). The inability to reveal a significant difference in this study may be attributed to the lack of a double-blinded design, and inadequate epilepsy diary recording [100]. Overall, these findings may provide novel perspectives in regards to using TQ as an adjuvant in antiepileptic therapies either to potentiate their actions or to alleviate their expected adverse effects. This may further worth investigating with contemporary AEDs. We sought in the present study to investigate the effects of TQ combined use with CBZ, a widely used AED, and to reveal the potential mechanism of action of TQ to either potentiate the therapeutic activity or reduce the onset of adverse effects in an epileptic model in mice.
CONCLUSION
Awareness of the value of the development of new therapeutic approach beyond the symptomatic treatment of epilepsy to modify the progression, or dare we suggest, prevent the development of epilepsy in the susceptible patient should be relentlessly increased, adherence to this activity not only leads to the strengthening of the rationality of drug therapy but also enhances drug safety in epilepsy. The realization of such a possibility will necessitate a change in our current AED discovery approach. Significant progress in our understanding of the factors that contribute to human epileptogenesis has been made in recent years. These advances have in large part been made possible through the development and characterization of genetic insult, and age-specific animal models of epileptogenesis and secondary neuronal hyperexcitability. The true validation of a given model of epileptogenesis requires the development of an effective therapy that prevents or delays the development of epilepsy or secondary neuronal hyperexcitability in the human condition, and whose activity was predicted by preclinical testing. Other key factors in the development of effective medication in epilepsy necessitate a greater understanding of the pathophysiology of epilepsy at the molecular and genetic level
It must be stressed that the principle prerequisite to unravel the basic aim to have a curative medication and to effectively control the resistant types of epilepsy, requires a precise and excellent assertive measure warranted to elucidate the mechanism of brain tissue glutamate reduction and enhancement of GABAergic mechanism in addition to modulation of vital ionic channels.
REFERENCES
- Patel MN. Oxidative stress, mitochondrial dysfunction, and epilepsy. Free Radic Res 2002;36(11):1139–46.
- Chen S-D, Chang AYW, Chuang Y-C. The potential role of mitochondrial dysfunction in seizure-associated cell death in the hippocampus and epileptogenesis. J Bioenerg Biomembr 2010;42(6):461–5.
- Rahman S. Pathophysiology of mitochondrial disease causing epilepsy and status epilepticus. Epilepsy Behav 2015;49:71–5.
- Noebels JL, Avoli M, Rogawski M, Olsen R, Delgado‐Escueta A V. “Jasper’s Basic Mechanisms of the Epilepsies” Workshop. Epilepsia 2010;51:1–5.
- Dhyani A, Kumar G. A New Vision To Eye: Novel Ocular Drug Delivery System. Pharmacophores. 2019 Apr 1;10(1):13-20.
- Rogawski MA, Löscher W. The neurobiology of antiepileptic drugs for the treatment of nonepileptic conditions. Nat Med 2004;10(7):685–92.
- Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeutics 2007;4(1):18–61.
- Buckmaster PS. Does mossy fiber sprouting give rise to the epileptic state? In: Issues in clinical epileptology: a view from the bench. Springer; 2014. page 161–
- Kempermann G. Adult neurogenesis: an evolutionary perspective. Cold Spring Harb Perspect Biol 2016;8(2):a018986.
- Antonucci F, Alpár A, Kacza J, Caleo M, Verderio C, Giani A, et al. Cracking down on inhibition: selective removal of GABAergic interneurons from hippocampal networks. J Neurosci 2012;32(6):1989–2001.
- Barker-Haliski M, White HS. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med 2015;5(8):a022863.
- Huberfeld G, Blauwblomme T, Miles R. Hippocampus, and epilepsy: Findings from human tissues. Rev Neurol (Paris) 2015;171(3):236–51.
- Clasadonte J, Dong J, Hines DJ, Haydon PG. Astrocyte control of synaptic NMDA receptors contributes to the progressive development of temporal lobe epilepsy. Proc Natl Acad Sci 2013;110(43):17540–5.
- Amakhin D V, Malkin SL, Ergina JL, Kryukov KA, Veniaminova EA, Zubareva OE, et al. Alterations in properties of glutamatergic transmission in the temporal cortex and hippocampus following pilocarpine-induced acute seizures in Wistar rats. Front Cell Neurosci 2017;11:264.
- Bertram EH. Temporal lobe epilepsy: where do the seizures really begin? Epilepsy Behav 2009;14(1):32–7.
- Vafaee F, Hosseini M, Hassanzadeh Z, Edalatmanesh MA, Sadeghnia HR, Seghatoleslam M, et al. The effects of Nigella sativa hydro-alcoholic extract on memory and brain tissues oxidative damage after repeated seizures in rats. Iran J Pharm Res IJPR 2015;14(2):547.
- Aguiar CCT, Almeida AB, Araújo PVP, Abreu RNDC de, Chaves EMC, Vale OC do, et al. Oxidative stress and epilepsy: literature review. Oxid Med Cell Longev 2012;2012.
- Khare CP. Indian medicinal plants: an illustrated dictionary. Springer Science & Business Media; 2008.
- Warrier PK. Indian medicinal plants: a compendium of 500 species. Orient Blackswan; 1993.
- Goreja WG. Black seed: nature’s miracle remedy. Karger Publishers; 2003.
- Datta AK, Saha A, Bhattacharya A, Mandal A, Paul R, Sengupta S. Black cumin (Nigella sativa L.)–a review. J plant Dev Sci 2012;4(1):1–43.
- Houghton PJ, Zarka R, de las Heras B, Hoult JRS. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med 1995;61(01):33–6.
- Ali BH, Al Za’abi M, Blunden G, Nemmar A. Experimental gentamicin nephrotoxicity and agents that modify it: a mini‐review of recent research. Basic Clin Pharmacol Toxicol 2011;109(4):225–32.
- Malik S, Ahmad S, Chaudhary I. Nigellimine N-oxide-a new isoquinoline alkaloid from the seeds of Nigella sativa. Heterocycles (Sendai) 1985;23(4):953–5.
- Malik S, Hasan SS, Choudhary MI, Ni C-Z, Clardy J. Nigellidine—a new indazole alkaloid from the seeds of Nigella sativa. Tetrahedron Lett 1995;36(12):1993–6.
- Malik S, Cun-Heng H, Clardy J. Isolation and structure determination of nigellicine, a novel alkaloid from the seeds of Nigella sativa. Tetrahedron Lett 1985;26(23):2759–62.
- Kumara SSM, Huat BTK. Extraction, isolation, and characterization of antitumor principle, α-hederin, from the seeds of Nigella sativa. Planta Med 2001;67(01):29–32.
- Nickavar B, Mojab F, Javidnia K, Amoli MAR. Chemical composition of the fixed and volatile oils of Nigella sativa L. from Iran. Zeitschrift für Naturforsch C 2003;58(9–10):629–31.
- Nobandegani AS, Motamedifar M. Antibiotic Sensitivity Profile of The Bacterial Isolates From The Blood Samples of The Patients In Different Wards of A Major Referral Hospital, Shiraz, Iran 2015-2016. Pharmacophores. 2019 Jun 1;10(2): 30-36.
- Sharma PC, Yelne MB, Dennis TJ. Database on medicinal plants used in Ayurveda. CCRAS New Delhi; 2005.
- Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity-the secret of Pharaohs: Therapeutic potential of black cumin seeds and beyond. Cancer Ther 2008;6(b):495.
- Yarnell E, Abascal K. Nigella sativa: holy herb of the Middle East. Altern Complement Ther 2011;17(2):99–105.
- Bakathir HA, Abbas NA. Detection of the antibacterial effect of Nigella sativa ground seeds with water. African J Tradit Complement Altern Med 2011;8(2).
- Morsi NM. Antimicrobial effect of crude extracts of Nigella sativa on multiple antibiotics-resistant bacteria. Acta Microbiol Pol 2000;49(1):63–74.
- Hannan A, Saleem S, Chaudhary S, Barkaat M, Arshad MU. Antibacterial activity of Nigella sativa against clinical isolates of methicillin-resistant Staphylococcus aureus. J Ayub Med Coll Abbottabad 2008;20(3):72–4.
- Salem EM, Yar T, Bamosa AO, Al-Quorain A, Yasawy MI, Alsulaiman RM, et al. Comparative study of Nigella Sativa and triple therapy in eradication of Helicobacter Pylori in patients with non-ulcer dyspepsia. Saudi J Gastroenterol Off J Saudi Gastroenterol Assoc 2010;16(3):207.
- Khan MAU, Ashfaq MK, Zuberi HS, Mahmood MS, Gilani AH. The in vivo antifungal activity of the aqueous extract from Nigella sativa seeds. Phyther Res An Int J Devoted to Pharmacol Toxicol Eval Nat Prod Deriv 2003;17(2):183–6.
- Randhawa MA. Black seed, Nigella sativa, deserves more attention. J Ayub Med Coll Abbottabad 2008;20(2):1–2.
- Mahmoud MR, El-Abhar HS, Saleh S. The effect of Nigella sativa oil against the liver damage induced by Schistosoma mansoni infection in mice. J Ethnopharmacol 2002;79(1):1–11.
- Mohamed AM, Metwally NM, Mahmoud SS. Sativa seeds against Schistosoma mansoni different stages. Mem Inst Oswaldo Cruz 2005;100(2):205–11.
- Bourgou S, Pichette A, Marzouk B, Legault J. Antioxidant, anti‐inflammatory, anticancer and antibacterial activities of extracts from Nigella sativa (black cumin) plant parts. J Food Biochem 2012;36(5):539–46.
- El Gendy S, Hessien M, Salam IA, Morad M, El-Magraby K, Ibrahim HA, et al. Evaluation of the possible antioxidant effects of Soybean and Nigella sativa during experimental hepatocarcinogenesis by nitrosamine precursors. Evaluation 2007;5:11.
- Sogut B, Çelik İ, Tuluce Y. The effects of diet supplemented with the black Cumin (Nigella sativa L.) upon immune potential and antioxidant marker enzymes and lipid peroxidation in broiler chicks. 2008;
- Salama RHM. Hypoglycemic effect of lipoic acid, carnitine, and Nigella sativa in diabetic rat model. Int J Health Sci (Qassim) 2011;5(2):126.
- Abdelmeguid NE, Fakhoury R, Kamal SM, Al Wafai RJ. Effects of Nigella sativa and thymoquinone on biochemical and subcellular changes in pancreatic β‐cells of streptozotocin‐induced diabetic rats. J Diabetes 2010;2(4):256–66.
- Kanter M, Akpolat M, Aktas C. Protective effects of the volatile oil of Nigella sativa seeds on β-cell damage in streptozotocin-induced diabetic rats: a light and electron microscopic study. J Mol Histol 2009;40(5–6):379–85.
- Nikakhlagh S, Rahim F, Aryani FHN, Syahpoush A, Brougerdnya MG, Saki N. Herbal treatment of allergic rhinitis: the use of Nigella sativa. Am J Otolaryngol 2011;32(5):402–7.
- Majdalawieh AF, Hmaidan R, Carr RI. Nigella sativa modulates splenocyte proliferation, Th1/Th2 cytokine profile, macrophage function, and NK anti-tumor activity. J Ethnopharmacol 2010;131(2):268–75.
- Ghonime M, Eldomany R, Abdelaziz A, Soliman H. Evaluation of immunomodulatory effect of three herbal plants growing in Egypt. Immunopharmacol Immunotoxicol 2011;33(1):141–5.
- El Daly ES. Protective effect of cysteine and vitamin E, Crocus sativus and Nigella sativa extract on cisplatin-induced toxicity in rats. J Pharm Belg 1998;53(2):87–93.
- Zaoui A, Cherrah Y, Mahassini N, Alaoui K, Amarouch H, Hassar M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 2002;9(1):69–74.
- Zedlitz S, Kaufmann R, Boehncke W-H. Allergic contact dermatitis from black cumin (Nigella sativa) oil-containing ointment. Contact Dermatitis 2002;46(3).
- El–Dakhakhny M. Studies on the chemical constitution of Egyptian Nigella sativa l. Seeds. Ii1) the essential oil. Planta Med 1963;11(04):465–70.
- Hirobe C, Qiao Z-S, Takeya K, Itokawa H. Cytotoxic principles from Majorana syriaca. Nat Med 生薬學雜誌 1998;52(1):74–7.
- Economakis C, Skaltsa H, Demetzos C, Soković M, Thanos CA. Effect of phosphorus concentration of the nutrient solution on the volatile constituents of leaves and bracts of Origanum dictamnus. J Agric Food Chem 2002;50(22):6276–80.
- Havlik J, Kokoska L, Vasickova S, Valterova I. Chemical composition of essential oil from the seeds of Nigella arvensis L. and assessment of its antimicrobial activity. Flavour Fragr J 2006;21(4):713–7.
- Salea R, Widjojokusumo E, Hartanti AW, Veriansyah B, Tjandrawinata RR. Supercritical fluid carbon dioxide extraction of Nigella sativa (black cumin) seeds using Taguchi method and full factorial design. Optimization 2013;13(14):16–7.
- Alhaj NA, Shamsudin MN, Zamri HF, Abdullah R. Extraction of essential oil from Nigella sativa using supercritical carbon dioxide: study of antibacterial activity. Am J Pharmacol Toxicol 2008;3(4):225–8.
- Alkharfy KM, Ahmad A, Khan RMA, Al-Shagha WM. Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. Eur J Drug Metab Pharmacokinet 2015;40(3):319–23.
- Sergeevna S M, Efimovna L E, Vladimirovna A I. Improvement of Pharmaceutical Consultation Process in Drugstores. J Adv Pharm Educ Res 2020;10(1):136-42
- Alsaleh N A. Pharmacist-led Flu Vaccination Services in Community Pharmacy: Experiences and Benefits. J Adv Pharm Educ Res. 2020;10(1):181-5.
- Ojha S, Azimullah S, Mohanraj R, Sharma C, Yasin J, Arya DS, et al. Thymoquinone protects against myocardial ischemic injury by mitigating oxidative stress and inflammation. Evidence-Based Complement Altern Med 2015;2015.
- Attia A, Ragheb A, Sylwestrowicz T, Shoker A. Attenuation of high cholesterol-induced oxidative stress in rabbit liver by thymoquinone. Eur J Gastroenterol Hepatol 2010;22(7):826–34.
- Nader MA, El-Agamy DS, Suddek GM. Protective effects of propolis and thymoquinone on development of atherosclerosis in cholesterol-fed rabbits. Arch Pharm Res 2010;33(4):637–43.
- Harzallah HJ, Kouidhi B, Flamini G, Bakhrouf A, Mahjoub T. Chemical composition, antimicrobial potential against cariogenic bacteria, and cytotoxic activity of Tunisian Nigella sativa essential oil and thymoquinone. Food Chem 2011;129(4):1469–74.
- Halawani E. Antibacterial activity of thymoquinone and thymohydroquinone of Nigella sativa L. and their interaction with some antibiotics. Adv Biol Res (Rennes) 2009;3(5–6):148–52.
- Umar S, Shah MAA, Munir MT, Yaqoob M, Fiaz M, Anjum S, et al. Synergistic effects of thymoquinone and curcumin on immune response and antiviral activity against avian influenza virus (H9N2) in turkeys. Poult Sci 2016;95(7):1513–20.
- Arslan SO, Gelir E, Armutcu F, Coskun O, Gurel A, Sayan H, et al. The protective effect of thymoquinone on ethanol-induced acute gastric damage in the rat. Nutr Res [Internet] 2005;25(7):673–80. Available from: http://www.sciencedirect.com/science/article/pii/S0271531705001090
- Bayrak O, Bavbek N, Karatas OF, Bayrak R, Catal F, Cimentepe E, et al. Nigella sativa protects against ischemia/reperfusion injury in rat kidneys. Nephrol Dial Transplant [Internet] 2008;23(7):2206–12. Available from: https://doi.org/10.1093/ndt/gfm953
- Vaillancourt F, Silva P, Shi Q, Fahmi H, Fernandes JC, Benderdour M. Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J Cell Biochem 2011;112(1):107–17.
- Pari L, Sankaranarayanan C. Beneficial effects of thymoquinone on hepatic key enzymes in streptozotocin–nicotinamide induced diabetic rats. Life Sci 2009;85(23–26):830–4.
- Isik AF, Kati I, Bayram I, Ozbek H. A new agent for treatment of acute respiratory distress syndrome: thymoquinone. An experimental study in a rat model. Eur J cardio-thoracic Surg 2005;28(2):301–5.
- Nagi MN, Alam K, Badary OA, Al‐Shabanah OA, Al‐Sawaf HA, Al‐Bekairi AM. Thymoquinone protects against carbon tetrachloride hepatotoxicity in mice via an antioxidant mechanism. IUBMB Life 1999;47(1):153–9.
- El-Khouly D, El-Bakly WM, Awad AS, El-Mesallamy HO, El-Demerdash E. Thymoquinone blocks lung injury and fibrosis by attenuating bleomycin-induced oxidative stress and activation of nuclear factor Kappa-B in rats. Toxicology 2012;302(2–3):106–13.
- Sayed‐Ahmed MM, Nagi MN. Thymoquinone supplementation prevents the development of gentamicin‐induced acute renal toxicity in rats. Clin Exp Pharmacol Physiol 2007;34(5‐6):399–405.
- Salehi P, Nasri S, Roghani M, Poordahandeh U, Baluchnejadmojarad T. The effect of thymoquinone on short-term spatial memory, passive avoidance learning, and memory of diabetic rats and the involvement of hippocampal oxidative stress. Pajoohandeh J 2012;17(5):219–27.
- Gali-Muhtasib H, El-Najjar N, Schneider-Stock R. Lead Molecules from Natural Products—Discovery and New Trends. 2006;
- Gökce EC, Kahveci R, Gökce A, Cemil B, Aksoy N, Sargon MF, et al. Neuroprotective effects of thymoquinone against spinal cord ischemia-reperfusion injury by attenuation of inflammation, oxidative stress, and apoptosis. J Neurosurg Spine 2016;24(6):949–59.
- Hosseinzadeh H, Parvardeh S. Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine 2004;11(1):56–64.
- Abdel-Fattah A-FM, Matsumoto K, Watanabe H. Antinociceptive effects of Nigella sativa oil and its major component, thymoquinone, in mice. Eur J Pharmacol 2000;400(1):89–97.
- Yajima Y, Narita M, Takahashi-Nakano Y, Misawa M, Nagase H, Mizoguchi H, et al. Effects of differential modulation of μ-, δ-and κ-opioid systems on bicuculline-induced convulsions in the mouse. Brain Res 2000;862(1–2):120–6.
- Ullah I, Badshah H, Naseer MI, Lee HY, Kim MO. Thymoquinone and vitamin C attenuates pentylenetetrazole-induced seizures via activation of GABA B1 receptor in adult rats cortex and hippocampus. Neuromolecular Med 2015;17(1):35–46.
- Shao Y, Li B, Huang Y, Luo Q, Xie Y, Chen Y. Thymoquinone attenuates brain injury via an antioxidative pathway in a status epilepticus rat model. Transl Neurosci 2017;8(1):9–14.
- Trinka E, Cock H, Hesdorffer D, Rossetti AO, Scheffer IE, Shinnar S, et al. A definition and classification of status epilepticus–Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015;56(10):1515–23.
- Shao Y, Feng Y, Xie Y, Luo Q, Chen L, Li B, et al. Protective effects of thymoquinone against convulsant activity induced by lithium-pilocarpine in a model of status epilepticus. Neurochem Res 2016;41(12):3399–406.
- Jokeit H, Schacher M. Neuropsychological aspects of type of epilepsy and etiological factors in adults. Epilepsy Behav 2004;5:14–20.
- Xie C, Sun J, Qiao W, Lu D, Wei L, Na M, et al. Administration of simvastatin after kainic acid-induced status epilepticus restrains chronic temporal lobe epilepsy. PLoS One 2011;6(9):e24966.
- Baluchnejadmojarad T, Roghani M. Coenzyme q10 ameliorates neurodegeneration, mossy fiber sprouting, and oxidative stress in intrahippocampal kainate model of temporal lobe epilepsy in rat. J Mol Neurosci 2013;49(1):194–201.
- Dariani S, Baluchnejadmojarad T, Roghani M. Thymoquinone attenuates astrogliosis, neurodegeneration, mossy fiber sprouting, and oxidative stress in a model of temporal lobe epilepsy. J Mol Neurosci 2013;51(3):679–86.
- Beyazcicek E, Ankarali S, Beyazcicek O, Ankarali H, Demir S, Ozmerdivenli R. Effects of thymoquinone, the major constituent of Nigella sativa seeds, on penicillin-induced epileptiform activity in rats. Neurosciences 2016;21(2):131.
- Raza M, Alghasham AA, Alorainy MS, El-Hadiyah TM. Beneficial interaction of thymoquinone and sodium valproate in experimental models of epilepsy: reduction in hepatotoxicity of valproate. Sci Pharm 2006;74(4):159–73.
- Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon C-S, Dykeman J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology 2017;88(3):296–303.
- Al Gharably NM, Badary OA, NAJI MN, Al Shabanah OA, Al Sawaf HA, AL RIKABI AC. Protective effect of thymoquinone against carbon tetrachloride-induced hepatotoxicity in mice. 1997;
- Daba MH, Abdel-Rahman MS. Hepatoprotective activity of thymoquinone in isolated rat hepatocytes. Toxicol Lett 1998;95(1):23–9.
- Nau H, Siemes H. Differentiation between valproate-induced anticonvulsant effect, teratogenicity, and hepatotoxicity. Pharm Weekbl 1992;14(3):101–7.
- Atack JR, Cook SM, Hutson PH, File SE. Kindling induced by pentylenetetrazole in rats is not directly associated with changes in the expression of NMDA or benzodiazepine receptors. Pharmacol Biochem Behav 2000;65(4):743–50.
- Mostafa RM, Moustafa YM, Mirghani Z. Thymoquinone alone or in combination with phenobarbital reduces the seizure score and the oxidative burden in pentylenetetrazole-kindled rats. Oxid Antioxid Med Sci 2012;1(3):185–92.
- Akhondian J, Parsa A, Rakhshande H. The effect of Nigella sativa L.(black cumin seed) on intractable pediatric seizures. Med Sci Monit 2007;13(12): CR555–9.
- Akhondian J, Kianifar H, Raoofziaee M, Moayedpour A, Toosi MB, Khajedaluee M. The effect of thymoquinone on intractable pediatric seizures (pilot study). Epilepsy Res 2011;93(1):39–43.
- Shawki M, El Wakeel L, Shatla R, Gamila E-S, Ibrahim S, Badary O. The clinical outcome of adjuvant therapy with black seed oil on intractable pediatric seizures: a pilot study. Epileptic Disord 2013;15(3):295–301.
