Physio-chemical Properties of Fresh, Clarified and Commercial Brands Pomegranate Juice
Lobna A. M. Haridy1, 2*, Maha A. Thaiban1, Aound Abed Alsrwani2
1 Department of Food and Nutrition, Faculty of Home Economics, King Abdulaziz University, Jeddah, Saudi Arabia.
2Food Technology Res. Inst. (FTRI). Agric.Res Center (ARC) 9 EL-Gamma St., Giza, Egypt.
*Email: Anood.abed @ outlook.com
ABSTRACT
Background : Juice can be considered as an important and functional ingredient in food products. Pomegranate (Punica granatum L.) juice (PJ) consumption has been increased recently due to scientific evidence increase on its high content of health beneficial compounds. The aim of the present study is to evaluate the physio-chemical properties of fresh, clarified and commercial pomegranate juice (commercial PJ). Materials and Methods : Four commercially available (labeled A, B, C and D), and one homemade (Clarified) and fresh pomegranate juice were evaluated for their physicochemical properties including total ash, pH value, titratable acidity (TA), total sugars (T.S), total soluble solids (T.S.S) and fruit percentage. Antioxidant properties such as anthocyanin, antioxidant activity (A.A) and total phenolic compound (TPC) were examined. Results : T.S.S and T.S at (P ≤ 0.05) were significantly higher in clarified PJ than those of all commercials and fresh juice by 19.3% and 16.9%, respectively. The control fresh PJ recorded the highest significant content of pH value (4.17 %) and lowest content of T.A% (6.4%) compared with commercial PJ sample B (32 %). TPC of commercial PJ samples B and C had 81.99 mg GAE/ml juice and 55.57 mg GAE/ml juice, respectively. The highest percentages of A.A were shown in the control fresh and clarified PJ sample as 84.5 % and 75.1%, respectively. Commercial PJ sample B recorded the highest content of anthocyanin (36.24 mg /100 ml) followed by commercial PJ samples C and D. The results ascertained that natural fresh PJ sample recorded the highest fruit percentage in juice (33.62%) followed by commercial PJ sample B and clarified PJ. Conclusion : The clarified PJ sample had the highest contents of T.S.S and total sugars than the control fresh PJ and all commercial PJ sample. Also, it was established that the control fresh PJ sample had a high antioxidant activity, fruit percentage and pH value, and low acidity. The commercial PJ sample B also contained a high percentage of total phenolic compounds and high percentage of total anthocyanin.
Key words: Pomegranate, Antioxidant Activity, Phenolic Compounds, Fruit percentage in juice.
INTRODUCTION
Fruit juices (100 % fruit) and nectars (25-99 % fruit) are consdiered as a valuable sector of the food industry with a global consumption volume of 36,247 millions litres in 2017. The 28 countries of the European Union (EU-28) accounted for 9,187 millions litres, followed by North America with 8,629 million litres, then the Asia-Pacific region with 8,159 million litres [1]. Pomegranate juice (PJ) has enjoyed considerable market growth and commercial success as a popular beverage in the United States and internationally for more than a decade [2]. According to 2014 estimates, 150,000-200,000 metric tons of fresh pomegranates and 3.7 millions gallons of pomegranate juice concentrate are sold annually [3]. Fresh PJ contains great amounts of total sugars, brix value, anthocyanins, vitamin C, phenols and proteins. It is considered as one of the richest sources of antioxidants [4]. Many clinical studies proved that PJ is useful to fight cancer, heart diseases, Alzheimer’s diseases and it changes different types of blood parameters including cholesterol, LDL and HDL [5, 6].
Carbonell-Barrachina [7] reported that pomegranate juice contains a special and high number of antioxidants. Meanwhile, [8, 9] ascertained that pomegranate juice is rich in elements including anthocyanins, ellagic tannins, gallic, catechins and ellagicacids, but the concentration and composition of phenol depends on the cultivar of the pomegranate. [10] mentioned that Taifi pomegranate seeds and fruit juice in Saudi Arabia contained moisture content by 79.28% and 84.57%, respectively. They also found that pomegranate had the highest content of total soluble solids (°Brix index). [11] found that T.S.S values of pomegranate juices were in the range between 14.79 to 15.81%.
According to [12], the total sugars of 30 sample Tunisian PJ ranged from 13.13–19.98 g/100mL. They found that fructose and glucose are the major sugars. Fructose ranged from 7.2–10.6 g/100mL (mean 9.05 g/100mL) and glucose ranged from 5.7–8.6 g/100mL (mean 7.28 g/100mL).
During pomegrante maturity, the change in composition of antioxidant activity is influenced by having bioactive compounds [13, 14]. Meanwhile, pomegranate juice showed high anti-oxidative properties due to its polyphenols, tannins and anthocyanins [15]. Furthermore, pomegranate juice contained a high antioxidant activity and was effective in the prevention of atherosclerosis [16]. Also, it was proved that PJ contained significantly more antioxidants than the other fruit juices and beverages [17]. The antioxidant activities for commercial pomegranate juices were found to be 10.4 and 67.5% by [18] and 18.6 and 42.8% by [19].
In addition, [20] stated that phenolic compounds are types of bioactive compounds. They reported that the TPC in juices from pomegranate taken and tested in three different stages of ripping from three varieties (Wonderful, Chaca and Codpa) recorded the highest content between 1.30 to 2.27 mg GAE / mL-1. Meanwhile, [18] reported that TPC ranged from 2.602 to 10.086 mg/mL1, some differences in phenolic composition between commercial pomegranate juices and experimental ones were observed by [21].
Minerals and total ash content in pomegranate (seeds and juice) were studied by [10]. The results showed that the percentages of ash in seeds and juice were 1.05 and 0.45%, respectively. The minerals with the highest amounts were calcium, sodium and potassium. They were recorded to be 24.8 mg/100 g, 76.1 mg/100 g and 307 mg/100 g in juice, respectively. These elements follow potassium, the most abundant element in the fruit.
According to [22], potassium is the predominant mineral found in pomegranate juice which ranges between 209.3 to 251.7 mg/100mL. They also added that Phosphorus of pomegranate juice ranged between 9.3to 15.1, Magnesium, 21to 104, Sodium 2.0 to 12.8 and Calcium 1.1 to 14.9 mg/100mL, respectively. Several published studies declare that the maximum pH of pomegranate juice (Taifi) is 3.57 at the stage of full-ripe and it increases with maturity. [10, 11, 23] stated that the values of pH in juice were in the range of 2.4 to 4.09 which are more than the values reported by [24]. The increased pH value leads to decrease of anthocyanin pigments [25]. On the other hand, pomegranate juice titratable acidity varied according to [22] which ascertained that it was ranged between 8.3 to 17.4%. [26] stated that the lower fruit content shows that there was addition of sugar and acids where bycitric acid is usually added to detect adulteration and ensure or mask the sugar addition [27]. According to [28], minimum percentage of fruit in juices should be 25%. Finally, pomegranate juice is considered to be one of the most important sources of anthocyanins given the fruit and aril its red color and some of phenolics and tannins such as punnicalin and ellagic acid [4]. Pomegranate has been considered being an interesting rich source of anthocyanins and other phenolic compounds [29].
MATERIALS AND METHODS
Materials
Fresh pomegranate fruits (Punica granatum) (Yemeni. sp), four different commercial brands pomegranate juices (PJs) namely, (A), (B), (C) and (D), and sucrose, and gelatin were bought from a market in Jeddah, the kingdom of Saudi Arabia. 2, 2-diphenyl-1-picrylhydrazyl (DPPH), Fructose, Glucose, and Sucrose were acquired from Sigma-Aldrich company, Jeddah. Folin-Ciocalteu’s phenol reagent was bought from scientific supply House, Jeddah. Sodium carbonate, Gallicacid, Sodium hydroxide, citricacid, potassium chloride, sodium acetate, Sodium benzoate, Hydrochloric acid, and Methanol were obtained from laboratory of food and nutrition department. The glass bottles were purchased from Bin Shahoun Commercial Center Al –balad, Jeddah, and Methanol was gotten from research facility of food and nutrition department. The glass containers were bought from Container Shahwan Commercial Center Al-balad, Jeddah.
Methods
Fresh pomegranates were weighted, cut with stainlesss teel knife and manually seeds, and peels were removed, then the percentage of edible portion, waste and juice were measured [30].
1. Technological methods
1.1. Preparationmaterial
The fruits were washed to remove the dusts and wiped completely to get dried, weighted, and cut into quarters with stainless steel knife and seeds (arils) were manually obtained from pomegranate fruits, then the percentage of edible portion of juice without waste was measured [30].
1.2. Technological processing of pomegranate juice
A. Extraction of fresh juice: The juice was extracted by blending the seeds in Juicer: BRAUN, type 4290. The obtained raw fresh juice was filtered by muslin cloth. The sterilized glass bottles were filled with the resultant juice and stored in refrigerator at 4±1ºC until use [31].
B. Clarification of juice: The juice was adjusted to pH value 3.0 and to 20% total soluble solids by adding citricacid and sucrose (3g citricacid /kg sucrose) using pH Meter: JENWAY, model: 3510. A solution of 1 % gelatin was added (10 % ml/kg juice) to precipitate tannins. Sodium benzoate (0.06%) was also added as a preservative material [32]. Then, the sterilized glass bottles were filled with the clarified juice and pasteurized in hot water bath (Water bath, JSR, model: jswb-11(T) and Refractometer: FISHER) for 1 min at 90°C to inactivate enzymes. The glass amber bottles were sealed, cooled and stored in refrigerator at 4°C ± 1 [31].
2 Analytical Methods
2.1 Physico-chemical analysis
A. Total ash content and pH value and total soluble solids (T.S.S%) were determined according to the methods of [33]. Total titratable acidity (T.T.A) was determined according to the method of [34]. Mineral analyses for juices were done according to the validated method of [35].
B. Total phenolic compounds (TPC): Total phenolic compounds’ concentrations in pomegranate juice were determined by the strategies of [36]. In brief, one millimeter of the sample (one milligram/ millimeter) was mixed with one millimeter of Folin-Ciocalteu’s phenol reagent. Ten millimeter of Na2CO3 (7%) solution was added to the mixture after 5 min. At that point, 13 millimeter of deionized water was included and mixed completely. The mixture was preserved within a dark environment for 90 min at 23°C, after which the absorbance (O.D.) was examined at 750 nm by spectrophotometer, Model: PD-303UV, APEL. The TPC was determined from extrapolation of calibration curve (Fig 1) which was made by preparing Gallicacid solution (Table 1). The evaluation of the phenolic compounds was conducted in triplicate. The results were recorded as mg of Gallicacid equivalents (GAE) per g of dried sample or per ml of juice sample.
Table 1. The series of different Gallic acid concentration
|
Concentration (ppm) |
Absorption(O.D) |
|
10 |
0.098 |
|
20 |
0.221 |
|
30 |
0.354 |
|
40 |
0.492 |
|
50 |
0.656 |
|
60 |
0.781 |
|
70 |
0.888 |
|
80 |
0.997 |
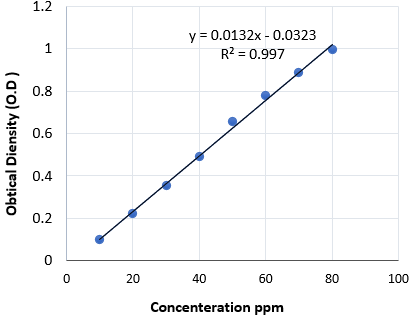
Figure 1. The standard curve of gallic acid
C. DPPH radical scavenging activity (Antioxidantactivity): Radical scavenging activity (RSA) was examined by 2.2-diphenyl-picrylhydrazyl radical (DPPH) according to the method performed by [37]. 0.5 ml of the sample was extracted with 15 ml of acid methanol by 1% HCL at room temperature for 1 hour. At that point, it centrifuged the extricate on 2500 rpm (Centrifugation: HERMLE, model: Z200A) for 15 minutes, the up layer was collected in measuring utensil. The collected extract adjusted pH value to reach (3.0). At that point, it was included to 50 ml methanol, 0.05 Or 1ml (new test) mixed with 5 ml of DPPH solution (0.025 g/L). The mixture and blank (1ml methanol blended with 5 ml DPPH solution were kept within the dark for 30 min at 23°C. The decrease in DPPH absorbance (A) was measured at 517 nm by spectrophotometer, Model: PD-303UV, APEL according to the blank (A0). The DPPH radical scavenging activity was calculated as: RSA, % = ([A0 – A]/ A0) × 100%.
D. Anthocyanins:
The pH differential strategy was portrayed by [38] using spectrophotometer, Model: PD-303UV, APPEL to decide to add up to monomeric anthocyanin concentration. 1 ml of the sample was extracted with 14 ml methanol (50%) by sonication for five minutes at that point centrifugation 4000 x g for 12 min (Centrifugation: HERMLE, model: Z200A). Then, 1 ml juice was diluted with 7 ml of sodium acetate buffer (pH 4.5) and potassium chloride buffer (pH 1.0)) pH Meter: JENWAY, model: 3510(.
Buffer preparation :
A. Sodium acetate buffer (pH 4.5) weighing 54.43 g CH3CO2Na*3H2O (Sodium acetate) was put in a beaker, then istilled water was added to ca 960 mL. The pH was adjusted to 4.5 (±0.05) with HCl (ca 20 mL). Then, it was moved to 1 L volumetric flask, and diluted to volume with refined water.
B. Potassium chloride buffer (pH 1.0) weighing 1.86 g KCl (Potassium chloride) was put in a beaker, then distilled water was added to ca 980 mL. The pH was adjusted to 1.0 (±0.05) with HCl (ca 6.3 mL). And it was moved to 1 L volumetric flask, and diluted to volume with distilled water, separately. After 10 min, the absorbance values of each buffer mixture was measured at 510 nm and 700 nm in a UV–visible spectrophotometer. It expressed the results as milligram cyanidin- 3-glucoside equivalent per 100 ml pomegranate juice (mg C3g E/100 ml PJ).
A = (A510 − A700) pH 1.0 − (A510 − A700) pH 4.5
Monomeric Anthocyanin Concentration (MAC) = A×MWDFε×L
Where A = absorbance values at 510 nm and 700 nm, ε = cyanidin-3- glucoside molar absorbance (26,900), MW = cyanidin-3-glucoside molecular weight (449.2 g/mol), DF = dilution factor, and L = cellpathlength (1 cm) [39].
E. The percentage of fruit in juice:
The percentage of fruit in juice was detected by the methods of [40]. 200 ml of the juice was concentrated in a porcelain dish on a water bath (JSR, model: jswb-11(T) at 80°C for 2 hours. It was neutralized to the pH 8 using firstly M and then 0.1 M sodium hydroxide (Dilute a non-standardized 1.0 M NaOH solution by a factor of 10 and thenstandardize). 20 ml of neutral 40 percent formaldehyde was added (formalin, previouslyneutralized to pH 8) and titrated to pH 8 using 0.1 M sodium hydroxide. The titer (ml) was divided by two representing the formaldehyde number (F) and the amount of orange juice in the sample (% m/v) is given by the expression (1. 05 F /1.4). The method can bereadily carried out using a pH meter (JENWAY, model: 3510(.
2.2 Qualitative and quantitative sugars analysis by High Performance Liquid Chromatography (HPLC) methods
0.01 g from each standard was added to 10 ml deionized water to obtain stock solution 1000 ppm; then, serial dilution was prepared and injected into HPLC and the peak areas obtained were plotted against each concentration [41].
(B) Sample preparation:
5 g of the sample was dissolved in about 40 ml HPLC water, mixed well and ultrasonic was considered for 15 min at 50 °C. Then, it was centrifuged for 10 min at 4000 rpm, the volume was adjusted to 50 ml with HPLC grade water and filtered through a 0.45 μm nylon membrane syringe filter [41].
3. Statisticalanalysis
All results were analyzed using Statistical Package for the Social Sciences (SPSS) for Windows, version 20 (SPSS Inc., Chicago, IL, USA). The collected data were displayed as mean± standard deviation (SD). Analysis of Variance (ANOVA) test was utilized for determining the significances among different groups. All differences were considered critical at P-values ≤ 0.05. [42].
The present investigation was designed to evaluate the physico-chemical properties of fresh, clarified and commercial brands pomegranate juices purchased from Saudi Arabia market. The obtained results could be showed and discussed under the following titles:
- Physico-chemical properties of fresh, clarified and brands commercial pomegranate juices
- Total Soluble Solids (T.S.S%):
In the present study, the results concluded that clarified pomegranate juice sample had the highest contents of Total Soluble Solids (T.S.S%) than the control fresh pomegranate juice and all commercial pomegranate juice samples (A, B, C and D) by the percentage of 36.78, 32.64, 29.53,16.06, 22.27, and 42.66%, respectively (Table 2.a and Fig. 2.a). This result is in agreement with the findings by [1, 28, 31].
Furthermore, commercial pomegranate juice samples C and D had a high percentage of T.S.S compared to fresh and all brands, this may be due to addition of sucrose during processing treatments. The T.S.S content of homemade juices was relatively stable (13.7–14.5ºBx) as mentioned by [43]; while, in the commercially available juices varied from 11.8 to 16.2º Bx. Other authors found that T.S.S% in fresh pomegranate juices reached to 14.94–14.04º Bx [44]. This variation may be due to the method of juice production, variety, growth region, growth year and maturity level of the fruit [45].
B. Total sugars
Additionally, the results concluded that clarified pomegranate juice sample had the highest contents of total sugars than the control fresh pomegranate juice and all commercial pomegranate juice samples (A, B, C and D) by the percentage of 35.5, 31.36, 23.07, 31.36, and 25.44 %, respectively (Table 2.a and Fig. 2.a). The results reported by [46] concluded that microfiltration clarified pomegranate juice had physicochemical and nutritional properties similar to those fresh juice. The results also concluded that commercial pomegranate juice D and B had a high percentage of total sugars than those of fresh and other brands.
C. pH value and acidity
The results illustrated that the control fresh pomegranate juice samplehad a higher content of pH value than those of clarified, commercial pomegranate juice samples (A, B, C and D) by the percentage of 22.54, 46.76, 36.69, 31.65, and 39.56%, respectively (Table 2.a and Fig. 2.a). These results were in agreement with previous results of [10] who reported that pH of pomegranate juice Taifi is 3.57 at the stage of full ripe and it increases with maturity.
Total titratable acidity in the control fresh pomegranate juice sample had a low content compared with those of all studied samples. This may be due to its freshness and high pH value. Furthermore, [47] reported that the content of acidity in PJ decreases in all levels of preservation, temperature of pasteurization and during storage interaction. The results also concluded that commercial pomegranate juice B had a high percentage of total titratable acidity than those of other brands. This may be due to its acidic taste and this is not desired by consumers.
Table 2.a. The physico-chemicalproperties of fresh, clarified and commercial pomegranate juices
|
(%) |
Samples |
Mean squares (MS). |
Standard Deviation S.D |
F (Calculated) statistic |
|||||
|
Juice (A) |
Juice (B) |
Juice (C) |
Juice (D) |
Fresh Juice (Control) |
Clarifiedjuice |
||||
|
Commercial |
|
|
|||||||
|
Total Soluble Solids (T.S.S) |
13.0 |
13.6 |
16.2c |
15.0c |
12.2 |
19.3a |
14.88 |
2.59 |
14.0727** |
|
Total sugars |
11.6 |
13.0b |
11.6 |
12.6c |
10.9 |
16.9a |
12.77 |
2.16 |
14.6854** |
|
2.22 |
2.64c |
2.85 |
2.52 |
4.17a |
3.23b |
2.94 |
0.49 |
14.6969** |
|
|
9.6c |
32a |
8.32 |
8.32 |
6.4 |
12.8b |
6.36 |
9.59 |
1.6245** |
|
|
**P≥0.01, Significance level P = 1.0000, DF=5 |
|||||||||
|
*as citric acid |
|||||||||
D. Total phenolic compounds, TPC
The results also ascertained that commercial pomegranate juice sample B had a high percentage of total phenolic compounds than those of control fresh, clarified, and commercial pomegranate juice samples (A, C and D) by the percentage of 42.66, 44.07, 51.12, 32.22, and 37.57%, respectively (Table 2.b and Fig. 2.b. The variability within the TPC values is caused by reactions happened through juice manufacture (i.e., hydroxylation, methylation, iso prenylation, imerization and/or glycosylation) [48]. The recent investigations by [43] revealed that the phenolic content in juices was strongly subordinate on the production process. The differences of pomegranate cultivars is the key factor in deciding the antioxidant activity and other physico-chemical characteristics [49].
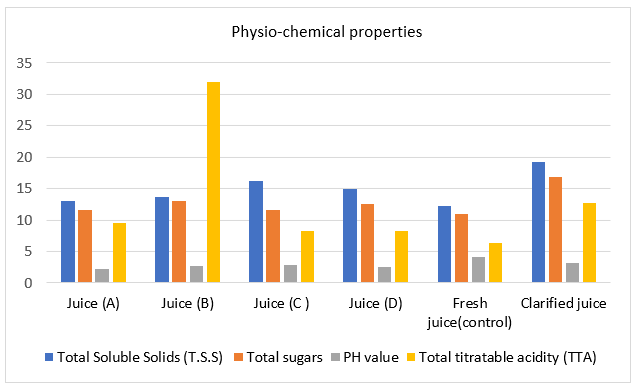
Figure 2.a. Histogram of the physico-chemicalproperties of fresh, clarified and commercial pomegranate juices
These differences came to the surface due to various reasons such as analytical methods, environmental conditions, cultivar and maturity stage as well as using Folin-Ciocalteure agent which overestimates the total individual phenolic compounds because of the interference with other reducing factors [50].
It was established that the control fresh pomegranate juice sample had a higher antioxidant activity than those of clarified, commercial pomegranate juice samples (A, B, C and D) by the percentage of 11.12, 89.34, 68.04, 42.01, and 56.21 %, respectively (Table 2.b and Fig. 2.b). The results also concluded that commercial pomegranate juice C and D had high percentage of antioxidant activity than those of other brands. These results were in agreement with the study of [51]. They revealed that using DPPH methods, antioxidant capacity of fresh pomegranate juices had higher antioxidant capacity than commercial pomegranate juice. This may be due to processing and pasteurization conditions that play an important role in the antioxidant capacity of juice, since the bioactive compounds are affected by extrinsic factors such as oxygen, light and especially temperature [52, 53]. Also, daily consumption of pomegranate juice is potentially a way better than apple juice in improving the antioxidant function within the elderly and increasing health benefits for the consumers [54].
F. Total anthocyanin
The results also ascertained that commercial pomegranate juice sample B had a high percentage of total anthocyanin than those of control fresh, clarified, commercial pomegranate juice samples (A, C and D) by 95.14, 83.85, 93.21, 67.77 and 85.48%, respectively (Table 2.b and Fig 2.b. These outcomes were in agreement with those of [55] who ascertained that the clarification and pasteurization during the production of fruit juices also influenced the steadiness of anthocyanins.The destruction of anthocyanin pigments increases with an increase in pH value depending on the cultivar, stage of maturity, clarification and pasteurization during the production of fruit juices [25, 56]. The important role of clarification is made clear when the pressing happens, for it helps in the reduction of tannin [57].
Vardin and Fenercioglu [58] declared the most appropriate clarification method due to preventing phenolic compounds reduction to a level that is acceptable, the decrease of turbidity, the preservation of anthocyanins and colordensity. This could be explained by dependence of the intensity and stability of the anthocyanin on juice pH [59].
G. Percentage of fruit in juice
The percentage of fruit in juice has indicated to be a useful parameter for detecting adulteration. The results also concluded that the control fresh pomegranate juice sample had a higher percentage of fruit in juice than those of clarified, and commercial pomegranate juice samples (A, B, C and D) by the percentage of 31.67, 66.53, 6.36, 73.88, and 78.28 %, respectively (Table 2.b and Fig 2.b). The lower fruit content shows that there was addition of sugar and acids where bycitric acid is usually added to detect adulteration and ensure or mask the sugar addition [27, 60]. The results also concluded that the control fresh pomegranate juice sample followed by commercial pomegranate juice sample B had nearly the same content of the percentage of fruit in juice approved in [28].
Table 2.b. The physicochemical properties of fresh, clarified and brands commercial pomegranate juices
|
Chemical constituents (%) |
Samples |
Mean squares (MS). |
Standard Deviation S.D |
F (Calculated) statistic |
|||||
|
Juice (A) |
Juice (B) |
Juice (C) |
Juice (D) |
Fresh Juice (Control) |
Clarifiedjuice |
||||
|
Commercial |
|
|
|||||||
|
totalPhenolic (mg GAE/ml) |
40.07 |
81.99a |
55.57b |
51.18c |
47.01 |
45.85 |
53.61 |
14.85 |
8.8489** |
|
Antioxidant Activity |
9 |
27 |
49c |
37 |
84.5a |
75.1b |
46.93 |
28.79 |
3.9943** |
|
Anthocyanin (mg /100 ml) |
5.26 |
5.85c |
10.54 |
13.07 |
5.9989 |
||||
|
19.23 |
11.72 |
6.0005 |
|||||||
|
**P≥0.01, Significance level P = 1.0000, DF=5, GAEmans Galic Acid Equivalent |
|||||||||
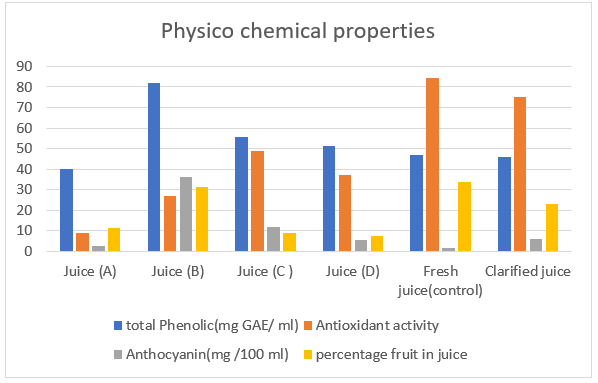
Figure 2.b. Histogram of physico-chemicalproperties of fresh, clarified and brands commercial pomegranate juices
- Ash and minerals of control fresh, clarified and brands commercial pomegranate juices
Commercial pomegranate juice sample B had a slightly high content of total ash by (0.5 %), followed by commercial pomegranate juice sample C by (0.2%). Whereas, fresh, clarified pomegranate juice and commercial pomegranate juice samples A and D had a low content of total ash. Meanwhile, commercial pomegranate juice sample B contained a high percentage of ash. Whereas, commercial pomegranate juice A, D and C contained a low percentage of ash. The results were in agreement with those of [10, 30]. Eight minerals were identified in all pomegranate juice samples (Table 3 and Fig. 3). The data indicated that there is a significant difference at (P ≤ 0.05) between minerals content of fresh, clarified and commercial pomegranate juice samples. Higher content concerning three elements (magnesium, phosphorus and potassium) shown in the control fresh pomegranate juice, clarified pomegranate juice and commercial pomegranate juice sample B (Table 3 and Fig. 3). The data in the same table also demonstrated that magnesium in the control fresh, clarified and commercial pomegranate juice sample B reached (4.7, 4.7, and 6.6, mg/100mL), respectively; but, phosphorus (10 ,10 and 12, mg/100mL), respectively. Finally, potassium was (113, 113 and157 mg/100 mL), respectively. Dominant minerals in commercial pomegranate juice sample (B) was potassium (157 mg/100mL) and in commercial pomegranate juice sample (C), it was phosphorus (26 mg/100mL). The amount of copper, iron, manganese and zinc were 0.5 mg/100 mL in all juices sample under study. Additionally, the results also showed thatpotassium and magnesium content of commercial pomegranate juice sample B, control fresh pomegranate juice and clarified pomegranate juice are higher than those of other juices. The results were in agreement with [61, 62]. The most common mineral in pomegranate juice was potassium according to the author [61].
Table 3. Ash and minerals of fresh, clarified and brands commercial pomegranate juices
|
Unit |
Samples |
Meansquares (MS). |
Standard Deviation S.D |
F (Calculated) statistic |
||||||
|
Juice (A) |
Juice (B) |
Juice (C) |
Juice (D) |
Freshjuice (Control) |
Clarifiedjuice |
|||||
|
Commercial |
|
|
||||||||
|
Ash |
g/10 mL |
0.1 |
0.5a |
0.2 b |
0.1 |
0.1 |
0.1 |
0.18 |
0.16 |
2.7557** |
|
Calcium |
1.0 |
7.0a |
3.0c |
4.0b |
2.0 |
2.0 |
3.17 |
1.29 |
6.0193** |
|
|
|
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.20 |
6.12** |
|
|
Iron |
|
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.20 |
6.12** |
|
|
1.2 |
6.6a |
2.1 |
2.2 |
4.7b |
4.7b |
3.58 |
1.46 |
6.0063** |
|
|
|
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.20 |
6.12** |
|
|
|
2.0 |
12b |
26a |
1.0 |
10.0c |
10.0c |
6.27 |
2.56 |
5.9993** |
|
|
|
27.0 |
157a |
41 |
26.0 |
113b |
113b |
79.5 |
32.46 |
5.9992 |
|
|
Zinc |
|
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.5 |
0.20 |
6.12** |
|
**P≥0.01 .Significancelevel P = 1.0000 .DF=5 According to the AIJN Reference Guide for PomegranateJuice(2007), the values of Ca, Mg and K shouldbe in the ranges 0.5–15, 2–10 and 80–250mg/100mL−1 respectively. Regarding the microelements (Fe ,Zn , Cu ,and Mn ) are alwaysbelow 0.5 mg/100mL. |
||||||||||
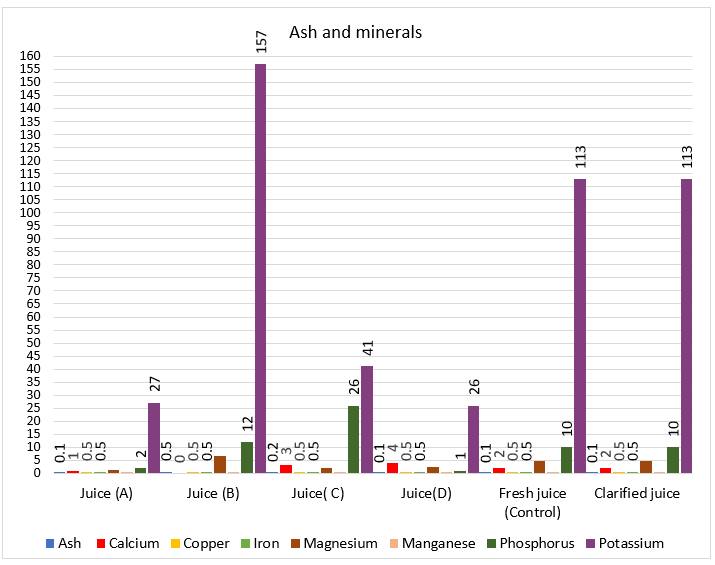
Figure 3. Histogram of ash and minerals of fresh, clarified and brands commercial pomegranate juices
3. Qualitative and quantitative sugars of fresh, clarified and brands commercial pomegranate juices which have been carried by HPLC:
Three fractionatedsugars, sucrose, glucose and fructose (Fig. 4) were analyzed as standards. Six investigated samples, including the control fresh pomegranate juice, clarified pomegranate juice and commercial pomegranate juices A, B, C, and D were analyzed. The concentration of fractionated sugars were expressed as g/100g (%).
The results of fractionated sugars, sucrose, glucose and fructose analysis concluded that commercial pomegranate juice sample D and C had a high content of sucrose by 11.3% and, 9.94%, respectively (Table 4 and Fig. 4) and low glucose and fructose. This may be due to adding sucrose during processing treatments of commercial pomegranate juice sample D and C. The trace and high concentration of sucrose in the PJ is possibly due to adulteration with another fruit juice like grape or peach juice [62, 63].
The results in the present study also concluded that control fresh pomegranate juice sample and clarified pomegranate juice sample had a high content of main fruits sugars (fructose) and glucose by 5.6 and 6.4%, respectively and glucose by 5.3 and 6.5%, respectively. The results are in agreement with those reported by [7]. They concluded that fructose and glucose were the main sugars found in the pomegranate juices and the differences could be related to fruit cultivar, climatic conditions and irrigation management, among other factors. Glucose and fructose were the dominant sugars detected by HPLC methods in all juices, according to the data reviewed by [7].
The present study also concluded that the control fresh and clarified PJ samples were free from sucrose, this may be due to its freshness. These results were in agreement with [60] and the Reference Guide for pomegranate juice [18]. Furthermore, the variation of very low sucrose content in pomegranate juice probably due to converted to invert sugars during the ripening process [12].
Table 4. Analysis of fractionated sugars of fresh, clarified and brands commercial pomegranate juices
|
(%) |
Samples |
Mean squares (MS). |
Standard Deviation S.D |
F (Calculated) statistic |
|||||
|
Juice (A) |
Juice (B) |
Juice (C) |
Juice (D) |
Freshjuice (Control) |
Clarified Juice |
||||
|
Commercial |
|
|
|||||||
|
Sucrose |
0.32 |
ND |
9.94b |
11.3a |
ND |
ND |
3.59 |
1.47 |
5.9821** |
|
5.27c |
5.93b |
1.60 |
5.3 |
6.5a |
4.27 |
1.74 |
6.0111** |
||
|
Fructose |
4.56 |
5.01c |
1.46 |
1.07 |
5.6a |
4.02 |
1.64 |
6.00** |
|
|
**P≥0.01 Significance level, P = 1.0000, DF=5 *ND: non detected. According to the AIJN Reference Guide for PomegranateJuice(2007), the values of fructose and glucose should be in the ranges 5.0–10 and 4.5–8 %respectively. |
|||||||||
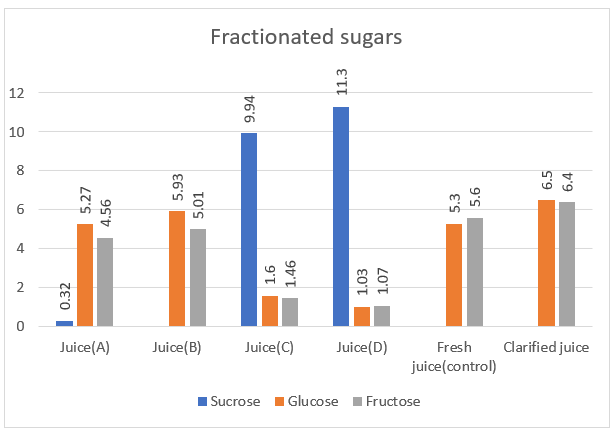
Figure 4. Histogram of fractionated sugars of fresh, clarified and brands commercial pomegranate juices
Figure 5 .Representative chromatograms of analyzed standard solution containing investigated sugars in mixture, 50.00 mm.sucrose, 8.867min; glucose, 10.617min; fructose, 13.650min. 300 mm x 7.8 mm operated at 80oC. flow rate 0.6 ml/min
Figure 6. HPLC chromatogram of analyzed juices containing sugars of fresh, clarified and brands commercial pomegranate juices, sucrose, 8.867min; glucose, 10.617min; fructose, 13.650min., 300 mm x 7.8 mm operated at 80oC. flow rate 0.6 ml/min.
CONCLUSION
Commercial pomegranate juice sample B contained a high percentage of total phenolic compounds, total anthocyanin, total ash, potassium, magnesium, total acidity, and percentage of fruit in juices. Commercial pomegranate juicesamples C and D had the lowest percentage of fruit in juices, low glucose and fructose, high content of sucrose, antioxidant activity and high T.S.S. Meanwhile, the commercial pomegranate juice samples A and B had a high content of glucose and fructose. The commercial pomegranate juice samples B and C had a higher content of total sugar than fresh pomegranate juice. The presence of low level of sucrose should be considered as an indicator of juice freshness. Furthermore, clarified pomegranate juice sample had the highest contents of T.S.S and total sugars than the control fresh pomegranate juice and all commercial pomegranate juice samples. Also, it was established that the control fresh pomegranate juice sample had a high antioxidant activity, fruit percenatge and pH value, and lowacidity. The control fresh and clarified pomegranate juice had a high level of potassium and magnesium after the commercial pomegranate juice sample B (The ash content is a measure of the total amount of minerals to assess the quality of the juice materials and potassium is the most abundant and characteristic mineral in pomegranate juice).
REFERENCES
- AIJNAIJF European Fruit juice Association - Annual report (2018) [Online]. Available: http://viewer.zmags.com/publication/bc62cfea#/bc62cfea/
- Robert Packe. Pomegranate juice adulteration, Food Safety Magazine. (2013) [Online]. Available https://www.foodsafetymagazine.com/signature-series/pomegranate-juice-adulteration/
- John, H. Adulteration of Pomegranate Products. A Review of the Evidence, 2016 ; (112): 62-69
- Kulkarni, A. P., Aradhya, S. M. Chemical changes and antioxidant activity in pomegranate arils during fruit development. Food Chemistry, 2005 ; 93(2), 319-32
- Sumner, M. D., Elliott-Eller, M., Weidner, G., Daubenmier, J. J., Chew, M. H., Marlin, R., Ornish, D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. The American journal of cardiology, 2005 ; 96(6), 810-814.
- Singh, M., Arseneault, M., Sanderson, T., Murthy, V., Ramassamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: bioavailability, metabolism, and cellular and molecular mechanisms. Journal of agricultural and food chemistry, 2008 ; 56(13), 4855-4873.
- Carbonell-Barrachina, A., Calín-Sánchez, A., Bagatar, B., Hernández, F., Legua, P., Martínez-Font, R., Melgarejo, P. Potential of Spanishsour–sweet pomegranates (cultivar C25) for the juice industry. Food science and technology international, 2012 ; 18(2), 129-138.
- Gil, M. I., Tomás-Barberán, F. A., Hess-Pierce, B., Holcroft, D. M., Kader, A. A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. Journal Of Agricultural and Food Chemistry, 2000 ; 48(10), 4581-4589.
- Borochov-Neori, H., Judeinstein, S., Tripler, E., Harari, M., Greenberg, A., Shomer, I., Holland, D. Seasonal and cultivar variations in antioxidant and sensory quality of pomegranate (Punica granatum L.) fruit. Journal of Food Composition and Analysis, 2009 ; 22(3), 189-195.
- Al-Maiman, S. A., Ahmad, D. Changes in physical and chemical properties during pomegranate (Punica granatum L.) fruit maturation. Food Chemistry, 2002 ; 76(4), 437-441.
- Abbas, F., Ali, N., Abbas, Y., Ali, A., Hussain, N., Abbas, T. Physicochemical Analysis of Pomegranate of Gilgit Baltistan, Pakistan. Agriculture, Forestry and Fisheries. 2015 ; 4(6) : 246-251.
- Hasnaoui, N., Jbir, R., Mars, M., Trifi, M., Kamal-Eldin, A., Melgarejo, P., Hernandez, F. Organicacids, sugars, and anthocyanins contents in juices of Tunisian pomegranate fruits. International Journal of Food Properties, 2011 ; 14(4), 741-757.
- Shwartz, E., Glazer, I., Bar-Ya’akov, I., Matityahu, I., Bar-Ilan, I., Holland, D., Amir, R. Changes in chemical constituents during the maturation and ripening of two commercially important pomegranate accessions. Food Chemistry, 2009 ; 115(3), 965-973.
- Fawole, O. A., Makunga, N. P., Opara, U. L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peelmethanolic extract. BMC complementary and alternative medicine, 2012 ; 12(1) : 200.
- Rosenblat, M., Hayek, T., Aviram, M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis, 2006 ; 187(2), 363-371.
- Li, Y., Guo, C., Yang, J., Wei, J., Xu, J., Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food chemistry, 2006 ; 96(2), 254-260.
- Seeram, N. P., Aronson, W. J., Zhang, Y., Henning, S. M., Moro, A., Lee, R. P., ... Pantuck, A. J. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. of Agricultural and Food Chemistry, 2008 ; 55(19), 7732-7737.
- Tezcan, F., Gültekin-Özgüven, M., Diken, T., Özçelik, B., Erim, F. B. Antioxidant activity and total phenolic, organicacid and sugar content in commercial pomegranate juices. Food Chemistry, 2009 ; 115(3), 873-877.
- Mousavinejad, G., Emam-Djomeh, Z., Rezaei, K., Khodaparast, M. H. H. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chemistry, 2009 ; 115(4), 1274-1278.
- Labbe, M., Ulloa, P. A., Lopez, F., Saenz, C., Pena, A., Salazar, F. N. Characterization of chemical compositions and bioactive compounds in juices from pomegranates (’Wonderful’, ’Chaca’and’Codpa’) at different maturity stages. Chilean Journal of Agricultural Research, 2016 ; 76(4), 479-486.
- Faria, A., Calhau, C. Pomegranate in human health: An overview. In Bioactive Foods in Promoting Health (pp. 551-563). Academic Press, 2010.
- Ekşi, A., Özhamamci, İ. Chemical composition and guide values of pomegranat ejuice. GIDA/The Journal of FOOD, 2009 ; 34(5).
- Cristosto, C., Mitcham, E., Kader, A. Pomegranate recommendations for main taining post harvest quality, Produce Facts Postuarthest Research and Iniforniation Center, University of California, Davis, USA, 2000 ; http: / / postharvest. ucdavis. edu / PFfruits / Pomegranate / (access on January 29, 2015)
- Cam, M., Hisil, Y., Durmaz, G. Characterisation of pomegranate juices fromten cultivars grown in Turkey. International Journal of Food Properties, 2009 ; 12(2), 388-395.
- Laleh, G. H., Frydoonfar, H., Heidary, R., Jameei, R., Zare, S. The effect of light, temperature, pH and species on stability of anthocyanin pigments in four Berberis species. Pakistan Journal of Nutrition, 2006 ; 5(1), 90-92
- Voldrich. M., Skalova. P., Kvasnicka. F., Cuhra. P., Kubik, M., Authenticity of 100% Orange Juice in the Czech Marketin 1996–2001, Journal of Food Science, 20002 ; 20 (2): 83-88
- Maireva, S., Manhokwe, S. The Determination of Adulteration in Orange Based Fruit Juices, International Journal of Science and Technology, 2013 ; 2(5): 365-372.
- Gcc Standariztion Organization. Codex General Standard for Fruit Juices and Nectars. Codex stan, 2015 ; 247.
- Seeram, N.P. Impact of pomegranate juice on human health and diseases .Nutritional Outlook, 2007 ; 235-237
- Usman, Saleemullah, Hafeezur, R.2, Sajjad, A., Zaid, K., Iftikhar, J., M Ayoub, K. Muhammad, H. Physio Chemical Properties of Pomegranate Varitieas Collected from Peshawar Local Market, agricultural research & technology Open Access Journal, 2018 ; 14(1):DOI 10-19080
- Nehal Rafik Abdel mohsen, chemical and technological studies on pomegranate fruit products, M.Sc D. Thesis, University of Cairo, Cairo, 2015, 106.
- Hamed, S. H. Some fundamental aspects affecting the processing of pomegranate juice. Egyptian Journal of Agricultural Research (Egypt), 1999 ; 77:1317-1327.
- AOAC, Official Methods of Analysis of AOAC. International 18th Edition, Maryland: Published by AOAC International, USA, 2007 ; 20877 – 2417.
- AOAC, Officialmethods of analysis (13th ed.) Washington, DC, USA: Association of OffcialAnalytical Chemists, 1980.
- Poitevin, E., Nicolas, M., Graveleau, L., Richoz, J., Andrey, D., Monard, F. Improvement of AOAC official method 984.27 for the determination of nine nutritional elements in food products by inductively coupled plasma-atomicemission spectroscopy after microwave digestion: Single-laboratory validation and ring trial. Journal of AOAC International, 2009 ; 92(5), 1484-1518.
- Saeed, N., Khan, M.R., Shabbir, M. Antioxidants activity, total phenolic and total flavonoied contents of whole plant extract storilisleptophylla L, BMC Complementary and Alternative Medicine, 2012 ; 12: 221.
- Su, M., Silva, J. Antioxidant activity, anthocyanins, and phenolics of rabbiteye (Vaccinium ashei) by-products as affected by fermentation. Food chemistry, 2006 ; 97 (3): 447-451.
- Giusti, M. M., Wrolstad, R. E. Characterization and measurement of anthocyanins by UV‐visible spectroscopy. Current Protocols in Food Analytical Chemistry, 2001 ; (1), F1-2.
- Mphahlele, R. R., Stander, M. A., Fawole, O. A., Opara, U. L. Effect of fruit maturity and growing location on the postharvest contents of flavonoids, phenolicacids, vitamin C and antioxid antactivity of pomegranatejuice (cv. Wonderful). Scientia Horticulturae, 2014 ; 179, 36-45.
- Kirk, S., Sawyer, R. Pearson's composition and analysis of foods (No. Ed. 9). (Longman Scientific &Technical, 1991 ; 708
- Castellari, M., Versari, A., Spinabelli, U., Galassi, S., Amati, A. An improved HPLC method for the analysis of organicacids, carbohydrates, and alcohols in grape musts and wines. Journal of Liquid chromatography & Related Technologies, 2000 ; 23(13), 2047-2056.
- Armitage, G.Y., Berry, W.G. Statistical Methods 7th Ed, Ames: Iowastate University press, 1987 ; 39-63.
- Dżugan, M., Wesołowska, M., Zaguła, G., Puchalski, C. The comparison of the physicochemical parameters and antioxidant activity of homemade and commercial pomegranate juices. Acta Scientiarum Polonorum Technologia Alimentaria, 2018 ; 17(1), 59-68.
- Ismail, F. A., Abdelatif, S. H., El-Mohsen, N. R. A., Zaki, S. A. The physico-chemicalproperties of pomegranate juice (Punica granatum L.) extracted from two Egyptian varieties. World Journal of Dairy& Food Sciences, 2014 ; 9(1), 29-35.
- Türkmen, İ., Ekşi, A. Brix degree and sorbitol/xylitol level of authenticpomegranate (Punica granatum) juice. Food Chemistry, 2011 ; 127(3), 1404-1407.
- Valero, M., Vegara, S., Martí, N., Saura, D. Clarification of pomegranate juice at industrial scale. Journal of Food Processing &Technology, 2014 ;5(5), 1.
- Suryawanshi, A.B., Kirad, K.S., Phad, G.N., Patil, S.B. Standardization of preservation method and their combination for safestorage of pomegranate juice at room temperature. The Asian Journal of Horticulture, 2008 ; 3: 395-399.
- Rice-Evans, C., Miller, N., Paganga, G. Antioxidant properties of phenolic compounds. Trends in plant science, 1997 ; 2(4), 152-159.
- Viyar, A. H., Qadri, R., Iqbal, A., Nisar, N., Khan, I., Bashir, M., Shah, F. Evaluation of unexplored pomegranate cultivars for physicochemical characteristics and antioxidant activity. Journal of Food Science And Technology, 2017 ; 54(9), 2973-2979.
- Pacini, C., Wossink, A., Giesen, G., Vazzana, C., Huirne, R. Evaluation of sustainability of organic, integrated and conventional farming systems: a farm and field-scale analysis. Agriculture, Ecosystems & Environment, 2003 ; 95(1), 273-288.
- Nuncio-Jáuregui, N., Cano-Lamadrid, M., Hernández, F., Carbonell-Barrachina, Á., Calín-Sánchez, Á. Comparison of fresh and commercial pomegranate juices from Mollar de Elche cultivar grown under conventional or organic farming practices. Beverages, 2015 ; 1(2), 34-44.
- Yildiz, H., Bozkurt, H., Icier, F. Ohmic and convention alheating of pomegranate juice: effects on rheology, color, and total phenolics. Food Science and Technology International, 2009 ; 15(5), 503-512.
- Gąstoł, M., Domagała-Świątkiewicz, I., Krośniak, M. Organic versus conventional–a comparative study on quality and nutritional value of fruit and vegetable juices. Biological Agriculture & Horticulture, 2011 ; 27(3-4), 310-319.
- Guo, C., Wei, J., Yang, J., Xu, J., Pang, W., Jiang, Y. Pomegranate juice is potentially better than apple juice in improving antioxidant function in elderly subjects. Nutrition Research, 2008 ; 28(2), 72-77.
- Turfan, Ö., Türkyılmaz, M., Yemiş, O., Özkan, M. Anthocyanin and colour changes during processing of pomegranate (Punica granatum L., cv. Hicaznar) juice from sacs and whole fruit. Food Chemistry, 2011 ; 129(4), 1644-1651.
- Zaouay, F., Salem, H. H., Labidi, R., Mars, M. Development and quality assessment of new drinks combining sweet and sour pomegranate juices. Emirates Journal of Food and Agriculture, 2014 ; 1-8.
- Dhinesh, K. V., Ramasamy, D. Pomegranate processing and value addition: review. Journal of Food Processing &Technology, 2016 ; 7(3), 565-566.
- Vardin, H., Fenercioglu, H. Study on the development of pomegranate juice processing technology: The pressing of pomegranate fruit. Acta Horticulture, 2009 ; 818: 373-381.
- Wilska-Jeszka, J., Korzuchowska, A. Anthocyanins and chlorogenic acid copigmentation-influence on the colour of strawberry and chokeberry juices. Journal of Food Examination and Research, 1996 ; 203(1), 38-42
- Singhal, R.S., Kulkami, P. R., Handbook of indices of foodquality and Authenticity (Woodhead, 2001), 561.
- AIJN (Association of the Industry of Juices and Nectars from Fruits and Vegetables of the EEC), Reference Guide for Pomegranate Juice. AIJN, Brussels, 2007.
- Nuncio‐Jáuregui, N., Calín‐Sánchez, Á., Hernández, F., Carbonell‐Barrachina, Á. A. Pomegranate juice adulteration by addition of grape or peach juices. Journal of the Science of Food and Agriculture, 2014 ; 94(4) : 646-655
- Fischer-Zorn, M., Ara, V. Pomegranate juice chemical composition and potential adulteration. Fruit Processing, 2007; 17 (4): 204-213.
