Predictors for the Diagnosis of Cardiac Tamponade in Patients with Pericardial Effusion
Fayez EL Shaer1,2*, Sami Idris1, Monir Hamed1, Anhar Ullah1, Rashed Alfagih1, Hamdan Al Shehri1,3
1 King Fahad Cardiac Center, Department of Cardiac Sciences, College of Medicine, King Saud University, Riyadh, Saudi Arabia,
2 National Heart Institute, Cairo, Egypt.
3Assistant Professor of internal medicine, Najran University, College of Medicine, Internal Medicine department, Saudi Arabia.
*Email: felshaer @ ksu.edu.sa
ABSTRACT
A controversial echocardiographic report is usually a warning alert about hemodynamic derangement; however, tamponade is almost always a clinical diagnosis which needs supplementation of echocardiographic finding. This study presents the diagnosis of cardiac tamponade in patients with pericardial effusion through clinical and echocardiographic (ECHO) assessment. Data for 72 patients were retrieved from case notes from King Fahad Cardiac Center (KFCC) from May 2015 to May 2019. Two-dimensional, M-mode, and Doppler readings were taken for every patient due to clinical instability. The diagnostic accuracy of different markers was measured with sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) via SAS/STATA software. Muffled heart sound, which is one of the criteria for tamponade was absent in 90% (n = 65) patients, while positive in only 10% (n = 7) patients. Echocardiographic examination pointed out the collapse of the right, atrium, or/and ventricle in 30 (42%) patients, whereas 42 (58%) patients had no collapse. The clinical Tamponade Risk Score (TRS) of more than 3 is highly suggestive of tamponade with a specificity of 100%, sensitivity of 76%, positive predictive value 100%, and negative predictive value of 88%. Findings indicated and corroborated conventional 2D ECHO can confirm the presence of pericardial effusion and evaluate the rise in intra-pericardial pressure by assessing the right heart chamber partial collapse even before blunting of hemodynamic parameters and settling clinical cardiac tamponade.
Key words: Cardiac Tamponade, Diagnosis, Predictors, Pericardial Effusion.
INTRODUCTION
Pericardial effusion is referred to as the accumulation of fluid throughout the pericardial space. It can be emerged in different medical conditions, majorly associated with cardiac surgery, and inflammation [1]. Cardiac tamponade is an extreme condition that prevails after the excessive rapid or sudden accumulation of fluid in the pericardial space that limits the adequate filling of the cardiac chambers. In particular, it disturbs normal hemodynamics and consequently causes cardiac arrest and hypotension. The occurrence of cardiac tamponade also exists when fluid accumulates massively in the pericardium, compromising hemodynamic. Therefore, it is a risky condition that must be diagnosed earlier for appropriate treatment and management [2].
Cardiac tamponade is rare as the earliest diagnosis of a malignancy. One of the most common sites from which cardiac metastases emerge is lung cancer. The occurrence of cardiac metastases usually prevails between 50 and 70 years through hematogenous and lymphatic dissemination. [3] The prevalence of cardiac tamponade is comparatively common in patients with malignant pericardial effusion even though its occurrence is low in the general population [4]. Primary pericardial tumors are not common such as cardiac tumors. They might be malignant or benign, with the prior being extremely unidentified. Various syndromes of cardiac compression or frank cardiac tamponade are caused by neoplastic pericarditis [5]. Tumor cells have the potential to regulate the coagulation system leading to hypercoagulable or prothrombic state. Malignancy can also be related to pulmonary embolism and deep venous thrombosis [6]. These pulmonary emboli might be another reason for cardiorespiratory suffering in cancer patients with hemodynamic decompensation [7].
The presence of a perforation or rupture of an intrapericardial organ leads to acute emergencies of cardiac tamponade [6]. Pericardial involvement can prevail even before the primary lesion, leading to tamponade.
The occurrence of asymptomatic pericardial effusion has been detected in approximately 100% of patients with dialysis and uremic pericarditis, leading from volume overload or pericarditis [8] In another study, pericardial effusion was detected in 62% of patients while 7.3% merely showed clinical or echocardiographic signs of tamponade [9].
It is essential to consider that progressive or persistent right heart failure is suggestive of cardiac tamponade when linked with hypotension. Pericardiocentesis can be done either ECHO guided, fluoroscopy-guided, or surgically. ECHO is an influential way of drainage in patients with cardiac tamponade and massive pericardial effusion. Using a percutaneous pigtail pericardial catheter can assist in achieving successful drainage.
This study, therefore, presents the diagnosis of cardiac tamponade in patients with pericardial effusion to highlight the challenges experienced in identifying this life-threatening condition.
MATERIALS AND METHODS
This study has reviewed all cases of pericardial effusion with hemodynamic instability, and /or cardiac tamponade in the cardiology department in King Fahad Cardiac Center (KFCC) from May 2015 to May 2019. Inclusion criteria include all patients who were >18 years, large (20 mm or more), and moderate (10-20 mm) pericardial effusion with heart compression. Patients with small effusion, chronic recurrent, and patients with effusive-constrictive pericarditis were excluded. Local anesthesia augmented by conscious sedation was provided to patients During pericardiocentesis. A limited lateral thoracostomy with the development of pericardial window has been done among a few patients with loculated pericardial effusion with background extensive adhesion. Data for 72 patients were retrieved from case notes. The following elements were noted including age, gender, clinical features, echocardiographic assessment, ECG, operative findings, investigative modalities.
Pericardial effusion was accounted to be moderate based on the echo fee distance anteriorly and posteriorly measuring between 10 and 20 mm, and large when the 20 mm limit is exceeded. The bedside clinical ground to define tamponade includes: increased venous pressure, decreased arterial pressure, increased heart rate above 100 bpm, and decreased intensity of heart sounds in the absence of any other possible explanation. The echocardiographic assessment was done with Philips IE 33 (Philips Ultrasound Bothell, WA, USA) cardiac ultrasound machine. The machine can perform two-dimensional, M-mode, continuous-wave Doppler, pulsed wave Doppler and color images via its variable frequency electronic transducer.
Unless impossible, due to patient clinical status, Inferior vena cava (IVC) two-dimensional M-mode and Doppler ECHO readings were taken for every patient. A dominant systolic venous flow that becomes more pronounced during expiration together with some diastolic flow reversal was considered as tamponade distinctive. Also, right atrial and/or ventricular collapse were considered when the right atrial collapse occurs during late diastolic and continuing till the first third of systole and during early to mid-diastole for the right ventricle. Pericardiocentesis was carried out when indicated.
Ethical approval from the Institutional Review Board (IRB) has been obtained before the study. The Tamponade Score (TS) was calculated as a combination of clinical, electrographic, and echocardiographic criteria for the diagnosis of clinically significant tamponade (on a score of 7 points). Simultaneous echocardiographic evaluation within 24 hours was carried out for every patient.
Categorical data were summarized with absolute numbers and percentages where continuous data were summarized as means and Standard Deviations (SD) or Median and Inter-quartile ranges (IQR). Comparisons between different groups were performed using the Chi-square test or Fisher’s exact test for categorical variables and t-test or Mann–Whitney U test for continuous variables. The diagnostic accuracy of different markers was measured with sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV). All the analysis was performed using [SAS/STATA] software (SAS Institute Inc., Cary, NC, USA).
RESULTS:
Overall, 72 patients (male: n = 30; female: n = 42) with moderate (n= 47; 65%) and large effusions (n=25; 35%) were included. Causes of cardiac tamponade were sporadic (idiopathic in 4% patients, tuberculosis in 1% patients, heart failure in 10% patients, traumatic in 8% patients, pericarditis in 7% patients, malignant in 18% patients, systemic lupus erythematosus (SLE) in 12.5% patients, renal failure in 15% patients, and post-cardiac surgery was detected in 22% patients). Dyspnea and chest pain were present in 75% (n = 54) and 24% (n = 17) patients, respectively (Table 1).
Table 1: Characteristics of the study group
|
|
TAMPONADE |
|
|||
|
Covariate |
Statistics |
Level |
No N=48 |
Yes N=24 |
P-value |
|
GENDER |
N (Col %) |
Female |
28 (58.33) |
14 (60) |
0.113 |
|
|
N (Col %) |
Male |
20 (41.66) |
10 (40) |
|
|
CHEST PAIN |
N (Col %) |
No |
38 (79.16) |
17 (70.83) |
0.459 |
|
|
N (Col %) |
Yes |
10 (20.83) |
7 (29.16) |
|
|
SOB |
N (Col %) |
No |
11 (22.91) |
7 (29.16) |
0.423 |
|
|
N (Col %) |
Yes |
37 (77.08) |
17 (70.83) |
|
|
HR |
N (Col %) |
No |
28 (59.57) |
12 (48) |
0.347 |
|
|
N (Col %) |
Yes |
19 (40.43) |
13 (52) |
|
|
BPS |
N (Col %) |
No |
47 (80.85) |
15 (62.5) |
0.077 |
|
|
N (Col %) |
Yes |
1 (2.12) |
9 (37.5) |
|
|
BPD |
N (Col %) |
No |
40 (83.33) |
19 (79.16) |
0.754 |
|
|
N (Col %) |
Yes |
8 (16.66) |
5 (20.08) |
|
|
HS muffled |
N (Col %) |
No |
48 (100) |
7 (29.1) |
<.001 |
|
|
N (Col %) |
Yes |
0 (0) |
6 (35.29) |
|
|
ELEC ALTE |
N (Col %) |
No |
46 (95.83) |
11 (45.83) |
0.004 |
|
|
N (Col %) |
Yes |
2 (4.16) |
13 (54.16) |
|
|
RV COLLA |
N (Col %) |
No |
46 (95.83) |
14 (58.33) |
<.001 |
|
|
N (Col %) |
Yes |
2 (4.16) |
10 (41.66) |
|
|
RA COLLP |
N (Col %) |
No |
40 (85.11) |
9 (37.5) |
<.001 |
|
|
N (Col %) |
Yes |
8 (16.66) |
15 (62.5) |
|
|
INTERVENTION |
N (Col %) |
No |
42 (87.23) |
6 (28) |
<.001 |
|
|
N (Col %) |
Yes |
6 (12.5) |
18 (72) |
|
|
RESP VARIATION |
N (Col %) |
No |
48 (100) |
10 (41.66) |
<.001 |
|
|
N (Col %) |
Yes |
0 (00) |
15 (62.5) |
|
|
AGE |
N |
|
47 |
25 |
0.637 |
|
|
Mean |
|
51.91 |
49.72 |
|
|
|
Median |
|
56 |
50 |
|
Muffled heart sound, which is one of the criteria for tamponade was absent in 90% (n = 65) patients, while positive in only 10% (n = 7) patients. Hypotension systolic blood pressure <100 mmHg was found in 10 (13.9%), and >100 mmHg in 62 (86%) patients. Electrical Alternans on ECG was found in 15 (21%) and absent in 57 (79%) patients.
Echocardiographic examination pointed out the collapse of the right, atrium, or/and ventricle in 30 (42%) patients, whereas 42 (58%) patients had no collapse. The venous flow was measured and analyzed in 48 (67%) patients; almost a quarter of them 11 (23%) had features compatible with tamponade. Significant Doppler respiratory variation (Mitral and Tricuspid) was present in 15 (21%) patients while absent in 57 (79%) patients (Figure 1). One-third of all patients (24) had cardiac tamponade based on bedside clinical ground (symptoms and signs of hemodynamic instability; tachycardia, hypotension, or distant heart sounds), while the other two-third (48) showed no clinical sign of cardiac tamponade (Figure 2).
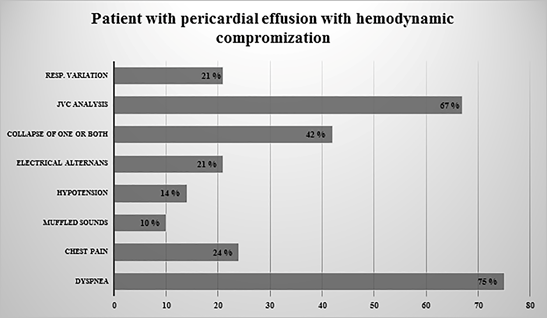
Figure 1: All Patients Clinical Characteristics
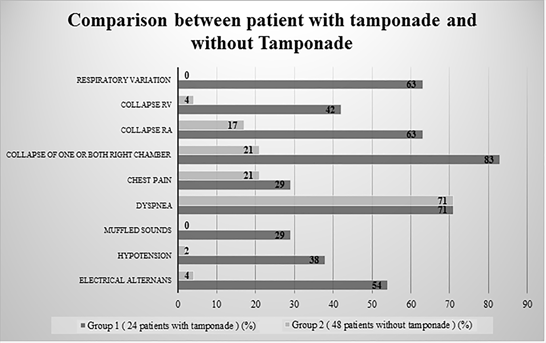
Figure 2: Comparison between a patient with tamponade and without Tamponade
Group 1: patients with Tamponade
24 Patients (males: 10; females: 14) with clinical features compatible with tamponade (group I) had a mean heart rate of 107+/-20. Of these, 15 subjects showed only moderate effusion while 9 patients had a large effusion. Dyspnea was present in 17 (71%) patients while chest pain was present in 7 (29%) patients. About 9 (37.5%) patients had systolic blood pressure < 100 mmHg and muffled heart sound was present in 7 (29%) patients. Electrical Alternant by ECG was found in 13 (54%) patients.
Only 5 out of 24 patients collapse for both right chambers, while 15 (63%) patients had collapse in one of the right chambers (Figure 3a). A total of 4 (16 %) patients showed no collapse at all. The right atrial collapse was present in 15 (63%) patients, while the right ventricular collapse was present in 10 (42%) patients (Figure 3b). Doppler respiratory variation was present in 15 (62.5%) patients, while absent in 9 (37.5%) patients. Pericardial effusion diameter was 2.0+/- 1.1 cm (Figure 3c).
Only 10 out of 24 patients showed inconclusive venous flow patterns due to its complications in analyzing the worst clinical status, which could not allow executing the proper echocardiographic study. The rest of the 14 (58%) patients had a dilated IVC, 1 patient showed a normal venous flow pattern depicted, 9 patients showed tamponades. Whereas, the diagnosis of 4 patients concerning venous flow morphology was questionable which might be partly explained because of the effusive constrictive pattern (Figure 3d). Overall, 24 patients showed immediate clinical improvement of their indices returned to normal after pericardial effusion drainage.
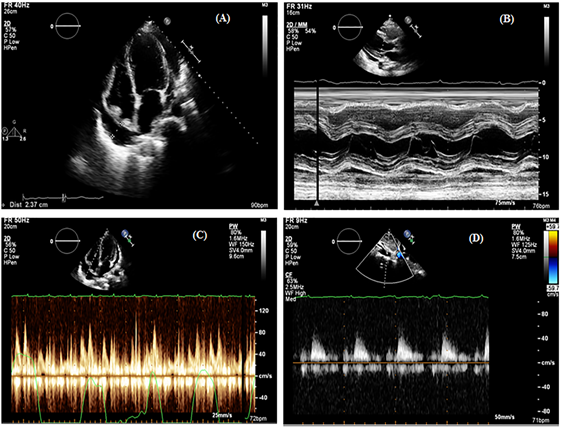
Figure 3: Right Chamber collapse (A) long parasternal M. Mode (B) Doppler respiratory variation (C) and (D) Venous flow in a patient with tamponade
Group 2 : patients without tamponade:
Among patients without clinical evidence of tamponade (group II), (48 patients; 20 men and 28 women), the Mean heart rate was 91+/- 19 beats per minute, effusion was moderate in 32 and large in 16 patients. Dyspnea was present in 37 (77%) patients while chest pain was present in 10 (21%) patients. Hypotension systolic blood pressure <100 was evident in one (2.1%) patient, while muffled heart sound was not recorded in those patients. Electrical Alternans was found in 2 (4.16%) patients by ECG.
The majority of the patients without clinical tamponade showed no apparent collapse when diagnosed with Doppler echocardiographic findings. Whereas, the collapse of one or more chambers was seen in 10 (21%) patients (right atrial collapse was seen in 8 (17%) patients and right ventricular collapse in 2 (4%) patients). The mean pericardial diameter was 2.4 ± 0.6 cm in patients without clinical evidence of tamponade. In this group, without clinical evidence of tamponade IVC was dilated and venous flow could be analyzed with normal morphology in 26 (76%), equivocal in 6 (18%), and suggestive of tamponade in 2 (6%) patients (Figure 2).
The diagnostic accuracy of different markers was measured with sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) as shown in Tables (2-4). The clinical Tamponade Risk Score (TRS) of more than 3 is highly suggestive of tamponade with the specificity of 100%, sensitivity of 76%, positive predictive value 100%, and negative predictive value of 88%.
Table 2: Specificities’ order
|
Parameter |
specificity |
positive predictive value (PPV) |
|
Muffled heart sound |
100% |
100% |
|
Respiratory variation |
100% |
100% |
|
Collapse of both RA&RV |
100% |
100% |
|
Hypotension less than 100 |
97.9% |
90% |
|
Electrical alternans |
95.8% |
86.6% |
|
Right ventricular collapse |
95.8% |
83.3% |
|
Venous flow pattern |
94%% |
81.8% |
|
Right atrial collapse |
83.3% |
65.5% % |
|
Pericardial diameter |
66.6% |
36 % |
PPV: positive predictive value
Table 3: sensitivities’ order
|
Parameter |
Sensitivity |
Negative predictive value (NPV) |
|
Venous flow pattern |
90% |
96.6% |
|
Respiratory variation |
62.5% |
84.2% |
|
Right atrial collapse |
62.5% |
81.6% |
|
Electrical alternans |
54.1% |
80.7% |
|
Right ventricular collapse |
41.6% |
76.6% |
|
Hypotension less than 100 |
37.5% |
75.8% |
|
Pericardial diameter |
37.5% |
68.2% |
|
Muffled heart sound |
29.1% |
73.8% |
|
Collapse of both RA& RV |
20% |
71.6% |
NPV: Negative predictive value
Table 4: Order of the specificities with other parameters:
|
Parameter |
specificity |
positive predictive value (PPV) |
sensitivity |
Negative predictive value (NPV) |
|
Muffled heart sound |
100% |
100% |
29.1% |
73.8% |
|
Respiratory variation |
100% |
100% |
62.5% |
84.2% |
|
Collapse of both RA&RV |
100% |
100% |
20% |
71.6% |
|
Hypotension less than 100 |
97.9% |
90% |
37.5% |
75.8% |
|
Electrical alternans |
95.8% |
86.6% |
54.1% |
80.7% |
|
Right ventricular collapse |
95.8% |
83.3% |
41.6% |
76.6% |
|
Venous flow pattern |
94%% |
81.8% |
90% |
96.65 |
|
Right atrial collapse |
83.3% |
65.5% % |
62.5% |
81.6% |
|
Pericardial diameter |
66.6% |
36 % |
37.5% |
68.2% |
DISCUSSION:
A controversial echocardiographic report is usually a warning alert about hemodynamic derangement; however, tamponade is almost always a clinical diagnosis which needs supplementation of echocardiographic finding, and the correlation between clinical and echocardiographic findings together with electrocardiographic findings to reach a score with better specificity and positive predictive value. The current study supported such a score which might be helpful to the clinician and will reflect on management, duration of hospital stays, and cost-effectiveness.
In patients with a significant amount of pericardial effusion, moderate to large, right ventricular and/or right atrial collapse is a common finding, particularly in patients with clinical presentation compatible with tamponade [10]. It is more common for the right atrium to show inversion than the right ventricle. Nevertheless, the right atrial collapse is not uncommon in patients with stable vitals, similarly, right ventricular collapse is far less common in patients without tamponade. Both concurrent inversions are quite rare if the patient is clinically stable [11].
The absence of a clinical feature of tamponade is strongly correlated with the absence of the right ventricle collapse. However, the right atrial collapse has a poor correlation with the clinical presentation [12]. Notably, the occurrence of atrial inversion in a vitally stable patient can be attributed to mild compression that does not have a significant ramification on chamber filling. When certain concomitant disease exists, hypovolemia, significant aortic incompetence, or left ventricular dysfunction, counting on the clinical presentation alone might be challenging [13, 14].
The present study results have indicated and corroborated that conventional 2D ECHO confirm the presence of pericardial effusion and evaluate the rise in intra-pericardial pressure by assessing the right heart chamber partial collapse even before blunting of hemodynamic parameters. Similarly, Clinical progression, as in the study by Levine et al. [15] could not be estimated because all the study patients with tamponade had undergone pericardial drainage. Previous studies [11-13] have tested the specificity, sensitivity, and positive and negative predictive values of collapse for tamponade diagnosis, with clinical tamponade as the reference standard [2, 16, 17]. In comparison to these studies, the sensitivity of any collapse was found to be remarkably low since right atrial, right ventricular, or both chamber collapse was separately considered. [18] Reydel et al. [12] have found a lower specificity of right atrial collapse and confirmed that right ventricular collapse is much more specific even though it represents one of the later manifestations of cardiac tamponade.
Furthermore, Spodick et al. [19] have indicated that intra-pericardial adhesion in organized effusion caused by infection might interfere with the development of collapse. Alternatively, localized tamponade was found to manifest only by compression of the vena cava or pulmonary veins [14]. This study has shown that the most sensitive echocardiographic features are those based on one or more chamber collapse and the most specific are those based on an abnormal respiratory variation of flow patterns [14]. Overall, such results advocate that the sensitivity of echocardiographic criteria of tamponade may be suboptimal.
In the present series of patients with moderate and large effusion, inferior vena cava flow evaluation was attempted in all patients. A major hindrance for such an assessment was the fact that flow was not feasible in all patients, some difficulties may be found if patients had atrial fibrillation, tricuspid regurgitation, pacemaker rhythm, ventilated patients, or postoperative patients [20-22]. In cardiac tamponade, venous flow pattern was predominantly systolic over the diastolic component that decreases during inspiration, and blunting or reverse of the diastolic component in the early expiratory cardiac cycle was found. The precise measurement of inferior vena cava flow was challenging in the current study due to patients’ instability secondary to decompensated cardiac tamponade.
Similarly, a study by Appleton et al. [23] has reported that inferior vena cava flow could be obtained in only 30% of cases. Burstow et al. [24] have also shown 50% of cases without clinical tamponade. Inferior vena cava flow was reported as equivocal in five patients and a constrictive pattern was noted in two patients. Overall, the inferior vena cava follow normalized after effusion was resolved. Abnormal inferior vena cava flow was exclusively observed in patients with chamber collapse. Similar results were depicted in the current study, where inferior vena cava flow was analyzable in two-third (n=48, 67%) of the patients (14 patients with clinical tamponade, and in 34 patients without clinical tamponade).
The present study showed that the prevalence of right-sided chamber inversion in patients with significant pericardial effusion, moderate to large, pointed to raised intra-pericardial pressure. However, this increased intra-pericardial pressure did not match clinical findings on many occasions. Similarly, clinical features alone sometimes may be of little help in patients with multiple co-morbid conditions and with other causes of clinical hemodynamic instability that could fail to pick up the right patients for intervention. Therefore, a combination of both parameters together with electrocardiographic findings raises the specificity and sensitivity for tamponade diagnosis and subsequent intervention.
Based on the existing data together with expert consensus, a stepwise scoring system for the triage of patients requiring pericardiocentesis was proposed. Sometimes cardiac tamponade grows slowly, and since signs and symptoms are neither sensitive nor specific, Halpern et al [25] have introduced a scoring index to help take clinical decision drainage. This index is based on effusion size, echocardiographic assessment, and clinical parameters. Furthermore, it should be applied in the absence of a shock. A total score of ≥6 warrants immediate intervention and drainage [26].
The current study has proposed a simplified combination score that can be of great help in guiding the therapy of cardiac tamponade patients. Furthermore, it is a promising tool, cost-effective, reduces hospitalization, and efficacious management. Therefore, this scoring system needs to be validated in large randomized trials with possible addition or deletion of some of the score points. This study is considered a platform for fostering such scores for further assessment. This study validated the specificity of such a combination score based on the highest specificity index. However, there were a relatively small number of patients in this study.
CONCLUSION
There is a good correlation between tamponade score and definitive cardiac tamponade, which is a significantly important tool for an accurate diagnosis. This score simplifies and unifies the clinical, echocardiographic and electrocardiographic, assessments into more robust relevant diagnoses that will enhance the management strategies with cost-effectiveness. Further studies are needed for better decision-making, particularly for invasive pericardial interventions. A score of three or more points diagnose cardiac tamponade with higher predictive accuracy for the below parameters and can be used for efficient diagnosis: (1) Muffled heart sound; (2) Respiratory variation; (3) RV collapse; (4) Electrical alternant; (5) Hypotension systolic blood pressure less than 100 mmHg; (6) Venous flow pattern suggestive of tamponade; and (7) RA collapse.
Conflicts of Interest
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
Funding Statement
The study is not funded through any source.
ACKNOWLEDGMENTS
The author is thankful to all the associated personnel, who contributed to this study by any means.
REFERENCES
- Chang EY, Ngo Q, Chaudhry AF, Sachdeva R. Rapidly Evolving Cardiac Tamponade In A Case Of Viral Pericarditis. InA56. CRITICAL CARE CASE REPORTS: CARDIOVASCULAR DISEASE I 2017 May (pp. A1934-A1934). American Thoracic Society.
- Pérez-Casares A, Cesar S, Brunet-Garcia L, Sanchez-de-Toledo J. Echocardiographic evaluation of pericardial effusion and cardiac tamponade. Frontiers in pediatrics. 2017 Apr 24;5:79. https://doi.org/10.3389/fped.2017.00079
- Molina JE. Pericardial effusions and cardiac tamponade. InCardiothoracic Surgical Procedures and Techniques 2018 (pp. 49-52). Springer, Cham. https://doi.org/10.1007/978-3-319-75892-3_10
- Rehman KA, Betancor J, Xu B, Kumar A, Rivas CG, Sato K, Wong LP, Asher CR, Klein AL. Uremic pericarditis, pericardial effusion, and constrictive pericarditis in end‐stage renal disease: Insights and pathophysiology. Clinical cardiology. 2017 Oct;40(10):839-46. https://doi.org/10.1002/clc.22770
- Dad T, Sarnak MJ. Pericarditis and pericardial effusions in end‐stage renal disease. InSeminars in dialysis 2016 Sep (Vol. 29, No. 5, pp. 366-373). https://doi.org/10.1111/sdi.12517
- Johnson MJ, Penmetcha R. Atypical presentation of STEMI with pericardial effusion causing cardiac tamponade related to malignancy. Br J Cardiol. 2019;26:159-60. https://doi.org/10.5837/bjc.2019.037
- Argula RG, Negi SI, Banchs J, Yusuf SW. Role of a 12‐lead electrocardiogram in the diagnosis of cardiac tamponade as diagnosed by transthoracic echocardiography in patients with malignant pericardial effusion. Clinical cardiology. 2015 Mar;38(3):139-44. https://doi.org/10.1002/clc.22370
- Matsakas EP, Lazaros GA, Panou FK, Karavidas AI, Papalimberi EP, Scotis ID, Zacharoulis AA. Primary pericardial fibrosarcoma presenting as “near” cardiac tamponade. Clinical Cardiology: An International Indexed and Peer‐Reviewed Journal for Advances in the Treatment of Cardiovascular Disease. 2002 Feb;25(2):83-5. https://doi.org/10.1002/clc.4950250210
- Jairath UC, Benotti JR, Spodick DH. Cardiac tamponade masking pulmonary embolism. Clinical cardiology. 2001 Jun;24(6):485-6. https://doi.org/10.1002/clc.4960240614
- Lorell, B. H. Pericardial diseases. In: Braunwald E, editor: Heart disease, A textbook of cardiovascular medicine. 5th ed. Philadelphia: WB Saunders Company, 1997; p. 1492.
- Plotnick GD, Rubin DC, Feliciano Z, Ziskind AA. Pulmonary hypertension decreases the predictive accuracy of echocardiographic clues for cardiac tamponade. Chest. 1995 Apr 1;107(4):919-24. https://doi.org/10.1378/chest.107.4.919
- Reydel B, Spodick DH. Frequency and significance of chamber collapses during cardiac tamponade. The American heart journal. 1990;119(5):1160-3. https://doi.org/10.1016/s0002-8703(05)80248-0
- Gentry J, Klein AL, Jellis CL. Transient constrictive pericarditis: current diagnostic and therapeutic strategies. Current cardiology reports. 2016 May 1;18(5):41. https://doi.org/10.1007/s11886-016-0720-2
- Fowler NO, Gabel MA. The hemodynamic effects of cardiac tamponade: mainly the result of atrial, not ventricular, compression. Circulation. 1985 Jan;71(1):154-7. https://doi.org/10.1161/01.cir.71.1.154
- Levine MJ, Lorell BH, Diver DJ, Come PC. Implications of echocardiographically assisted diagnosis of pericardial tamponade in contemporary medical patients: detection before hemodynamic embarrassment. Journal of the American College of Cardiology. 1991 Jan 1;17(1):59-65. https://doi.org/10.1016/0735-1097(91)90704-d
- Fadel BM, Galzerano D, Pergola V, Di Salvo G. Massive pericardial effusion without cardiac tamponade. European heart journal. 2016 Sep 1;37(33):2612-. https://doi.org/10.1093/eurheartj/ehw076
- Mahajan K, Asotra S, Negi P, Gupta G. Massive right pleural effusion leading to cardiac tamponade in absence of pericardial effusion: a rare presentation. Case Reports. 2016 Feb 3;2016:bcr2015214342. https://doi.org/10.1136/bcr-2015-214342
- Appleton C, Gillam L, Koulogiannis K. Cardiac tamponade. Cardiology Clinics. 2017 Nov 1;35(4):525-37. https://doi.org/10.1016/j.ccl.2017.07.006
- Spodick DH. The normal and diseased pericardium: current concepts of pericardial physiology, diagnosis and treatment. Journal of the American College of Cardiology. 1983 Jan 1;1(1):240-51. https://doi.org/10.1016/s0735-1097(83)80025-4
- Welch TD, Oh JK. Constrictive pericarditis. Cardiology clinics. 2017 Nov 1;35(4):539-49. https://doi.org/10.1161/circulationaha.106.635433
- Lorini FL, Cerutti S, Di Dedda G. Pericardium and Pericardial Disease. InTextbook of Echocardiography for Intensivists and Emergency Physicians 2019 (pp. 117-123). Springer, Cham. https://doi.org/10.1007/978-3-319-99891-6_10
- Chetrit M, Mardigyan V. Pericardial diseases. InCase-Based Textbook of Echocardiography 2018 (pp. 267-278). Springer, Cham. https://doi.org/10.1007/978-3-319-67691-3_20
- Appleton CP, Hatle LK, Popp RL. Superior vena cava and hepatic vein Doppler echocardiography in healthy adults. Journal of the American College of Cardiology. 1987 Nov 1;10(5):1032-9. https://doi.org/10.1016/s0735-1097(87)80343-1
- BURSTOW DJ, OH JK, BAILEY KR, SEWARD JB, TAJIK AJ. Cardiac tamponade: characteristic Doppler observations. InMayo Clinic Proceedings 1989 Mar 1 (Vol. 64, No. 3, pp. 312-324). Elsevier. https://doi.org/10.1016/s0025-6196(12)65251-3
- Halpern DG, Argulian E, Briasoulis A, Chaudhry F, Aziz EF, Herzog E. A novel pericardial effusion scoring index to guide decision for drainage. Critical pathways in cardiology. 2012 Jun 1;11(2):85-8.. https://doi.org/10.1097/hpc.0b013e318254a5ca
- Imazio M, Mayosi BM, Brucato A, Markel G, Trinchero R, Spodick DH, Adler Y. Triage and management of pericardial effusion. Journal of Cardiovascular Medicine. 2010 Dec 1;11(12):928-35. https://doi.org/10.2459/jcm.0b013e32833e5788.
