The Influence of Constant Lighting on The Daily Dynamics of Some Physiological Parameters of Male Wistar Rats
Lyudmila Makartseva, Maria Kozlova, Sergey Kucher, David Areshidze*
1 Researching Laboratory of Experimental Biology and Biotechnology, Moscow State Regional University, 10A Radio st., Moscow, Russian Federation.
*Email: notbio @ mgou.ru
ABSTRACT
The effect of the stay of 6-months-old male Wistar rats under the influence of constant lighting on the daily dynamics of some biochemical parameters was studied. Male Wistar albino rats at the age of 6 months were divided into 2 groups. The rats from the Group I were housed under a fixed illumination, L:D 14:10 (±180 lux, respectively; 8:00 AM lights on) (unless mentioned otherwise) in a temperature-controlled environment with ad libitum access to tap water and food (rat chow). Animals of Group II were studied under the same experimental conditions except for the light regime, representing constant light (LL ±180 lux). Both the first and second groups of animals were kept at the specified light regime for 2 weeks. A number of biochemical parameters of blood plasma have a reliable daily rhythm, while for other metabolites of an organism a circadian rhythm is not noted. The appearance of the previously absent rhythm of physiological parameters after the application of constant lighting indicates the role of other pacemakers in the metabolism, in addition to illumination. The conducted research testifies about the unambiguous modulating influence of constant illumination on the structure of diurnal dynamics of studied biochemical parameters and rectal temperature, and about the initiation of desynchronosis by it.
Key words: photoperiod, desynchronosis, circadian rhythm, rectal temperature, glucose.
INTRODUCTION
Evolutionarily determined endogenous daily rhythm or circadian rhythm (CR) is a characteristic property of living organisms. The molecular genetic mechanisms of circadian biological rhythms under various conditions can be implemented at the one-cell level [1]. Among the environmental factors affecting daily rhythm, the leading is photoperiodicity (the duration of daily and/or seasonal illumination). Photoperiodicity is the main pacemaker (time-setting cue or external synchronizing factor) for mammals [2-4].
The range of circadian rhythm fluctuations is 24±4 hours and depends on the intensity and period of external illuminance. In the case of the absence of changes in external illuminance circadian rhythms become free-running, and the characteristic of their period becomes different from 24 hours [5-9]. For multicellular animals, as their organization level becomes more complex, the significance of central oscillators regulating the general harmony of rhythms increases, but at the same time, the mechanisms of maintaining rhythmic processes in peripheral tissues and organs become more complicated [10].
In modern science, the concept of a multi-oscillatory model of regulation of the circadian system of mammals, where the suprachiasmatic nuclei of the hypothalamus act as the central oscillator and the pineal gland represents the endocrine modulator [11], is most widely accepted. Moreover, this concept does not detract the role of peripheral mechanisms of the regulation of circadian processes in organs [12-14].
Now, a fairly large number of people are exposed to light at nighttime. This impact may be associated with the profession or maybe due to habit and lifestyle. The continual exposure to excessive lighting is an essential part of the modern mode of life and is accompanied by many serious behavioral and health disorders. Exposure to light at night disrupts the endogenous circadian rhythm and inhibits the nighttime secretion of melatonin by a pineal gland, which leads to a decrease in its blood concentration [15, 16]. Inhibition of a function of the pineal gland due to exposure to constant light contributes to carcinogenesis, while the absence of light inhibits carcinogenesis [17-19]. Disruption of CR during shift working leads to an increased risk of cardiovascular diseases, metabolic syndrome, and type II diabetes mellitus [20]. Animal studies testify that replacing of total darkness with low lighting at night causes metabolic disorders and obesity [21-23].
It is certain that staying in conditions of constant light leads to the appearance of external desynchronosis, which means the pathological condition of the organism that occurs under the action of an extreme factor and is characterized by desynchronization of biorhythms. Desynchronosis resulting from constant illumination, like other types, has several stages and a number of common syndromes.
Stages of desynchronosis: I – stage of temporal dissonance; II – stage of regulatory disorders; III – stage of energetic or biochemical disorders; IV – stage of structural disorders in the source of oscillation and period of biorhythms. Syndromes of desynchronosis: 1) asthenoneurotic – disruption of rhythms of the cerebral cortex (headache, weakness, fatiguability); 2) neural-dystrophic – disruption of rhythms of subcortical structures and hypothalamus; 3) vegetative disorders – disruption of rhythms of the vegetative nervous system; 4) endocrine disorders.
To obtain more complete information about the manifestations of desynchronosis, we found it relevant to study the long-term effect of constant lighting on the complex of some biochemical parameters and dynamic of the rectal temperature of Wistar rats.
MATERIALS AND METHODS
Animals
The research was conducted on male Wistar albino rats at the age of 6 months. Animals were taken from the Stolbovaya nursery (the "Stolbovaya" affiliate of the Federal State Budgetary Institution of Science "Scientific Center for Biomedical Technologies of the Federal Medical and Biological Agency). The study involved two groups of animals: Group I (fixed light) and Group II (constant light). Each group included 32 animals.
Treatment design
During the whole experiment, the rats from the Group I were housed under a fixed illumination, L:D 14:10 (±180 lux, respectively; 8:00 AM lights on)(unless mentioned otherwise) in a temperature-controlled environment with ad libitum access to tap water and food (rat chow).
Animals of Group II were studied under the same experimental conditions except for the light regime, representing constant light (LL ±180 lux). Both the first and second groups of animals were kept at the specified light regime for 2 weeks. After two weeks, euthanasia of the animals in the carbon chamber was performed at 9 hours, 15 hours, 21 hours, and 3 hours; blood samples were taken for biochemical studies.
All animal experiments were performed according to the compliance with EC Directive 86/609/EEC and with the Russian law regulating experiments on animals.
Biochemical studies
In the blood plasma, using the StatFax-3300 (USA) analyzer with appropriate «Spinreact» kits (Spain), the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein, albumin, glucose, uric acid, creatinine, and alkaline phosphatase were determined.
Rectal temperature studies
For the measurement of rectal temperature, the BIO-TK8851 thermometer (Bioseb, USA) was used.
Statistical Analysis
The obtained data, analyzed using Graph Pad Prism 6.0, were expressed as Mean±SD. For the analysis of the characteristics of the circadian rhythm of the studied substances, the cosinor analysis carried out by means of the Cosinor Ellipse 2006-1.1 program was used. The presence of a reliable circadian rhythm and also its acrophase and amplitude were determined. Acrophase is the measure of peak time of the total rhythmic variability in a 24-hour period. Amplitude corresponds to half of the total rhythmic variability in a cycle. The acrophases are expressed in hours; amplitude values are expressed with the same units as the studied parameters.
RESULTS AND DISCUSSION
As a result of the conducted research, it is established that at fixed light regime, the rectal temperature dynamics at male rats of 6 months age are smoothed, wherein the temperature reaches its maximum value at night (Fig. 1). Keeping of animals at the regime of constant light leads to the fact that the maximum temperature is noted at 2100 hours, and at nighttime, in opposition to the values of the control, this parameter is minimal.
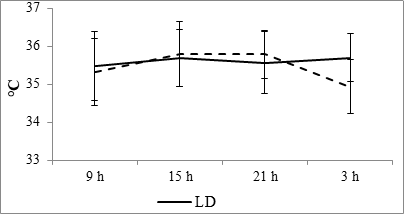
Fig. 1. Daily dynamics of the rectal temperature of rats at fixed light regime (LD) and constant illumination (LL).
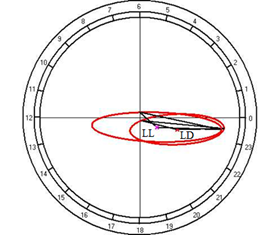
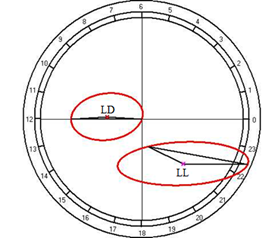
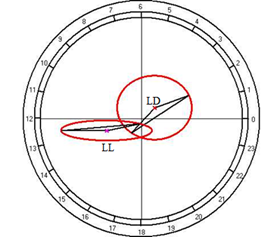
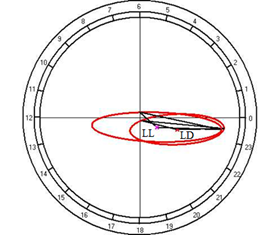
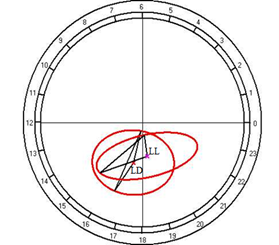
According to the results of cosinor analysis, the presence of the circadian rhythm of temperature with an amplitude of 2.02°C and an acrophase at 1502 was established at the fixed light regime, but at the regime of constant light a reliable circadian rhythm was not noted (Fig. 10A).
The diurnal dynamics of glucose values at the fixed light regime, like the dynamic of temperature, has a smoothed format with a maximum in the morning and a minimum at night. At the regime of constant lighting, as well as in the case of temperature, the minimum values of the parameter are noted at that time when there was a maximum at the fixed light regime (Fig. 2).
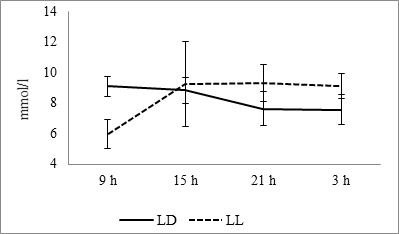
Fig. 2. Daily glucose dynamics in the blood plasma of rats at fixed light regime (LD) and constant illumination (LL).
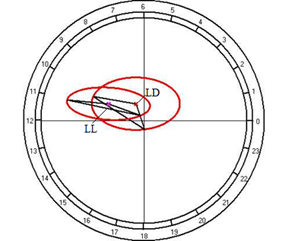
As the results of cosinor analysis, it is established the presence of reliable circadian rhythm for both of the light regimes. At the fixed light, the rhythm is characterized by acrophase falling on 1142 hours and amplitude of 0.96 mmol/l, and the regime of constant light acrophase shifts on 2216 hours with an increase of amplitude up to 1.68 mmol/l (Fig. 10B).
The analysis of the dynamics of the total plasma protein at the fixed light regime makes it possible to establish its minimum values in the daytime and maximum at night. Changing of the lighting regime leads to the inversion of the daily dynamics – the maximum and minimum times are in antiphase relative to those for a fixed lighting regime (Fig. 3).
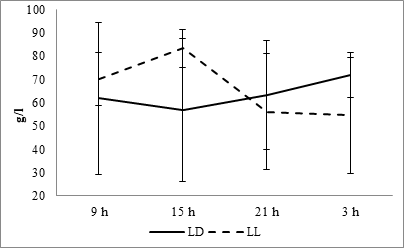
Fig. 3. Daily dynamics of total protein in the blood plasma of rats at fixed light regime (LD) and constant illumination (LL).
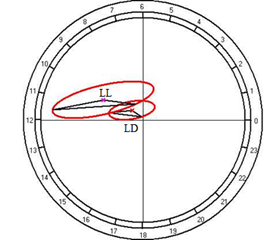
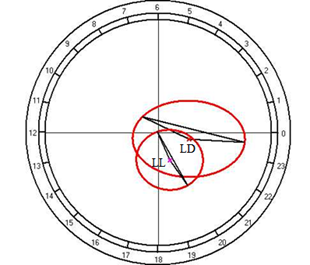
The cosinor analysis demonstrated the absence of a reliable CR at fixed light regime, but in the conditions of constant light, it appears and is characterized by the amplitude of 16.04 g/l and acrophase at 1314 hours (Fig. 10C).
The examination of the diurnal dynamic of albumin values at a fixed light regime revealed the presence of a maximum at 0300 and a minimum at 1500. At the same time at the regime of constant light, there is the rhythm inversion – the maximum occurs at 1500 hours, and the minimum – at 3 hours (Fig. 4).
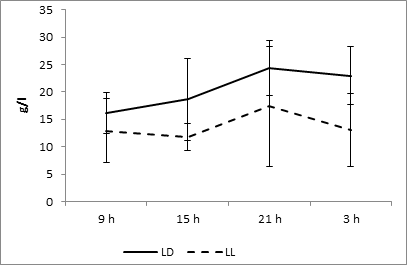
Fig. 4. Daily dynamics of albumin in the blood plasma of rats at fixed light regime (LD) and constant illumination (LL).
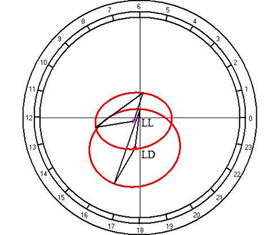
Cosinor analysis did not reveal the reliable circadian rhythmicity of this metabolite in both studied regimes (Fig. 10D).
At fixed light regime, the maximum level of uric acid was noted at 0900 hours and minimum – at 1500 hours. At the regime of constant light, the maximum level is also noted at 0900 hours, but the minimum falls at 2100 hours (Fig. 5). Cosinor analysis shows the absence of reliable rhythm at fixed light regime, but in conditions of constant light, it appears. Its amplitude makes 67.19 µmol/l, and acrophase falls at 1016 hours (Fig. 10E).
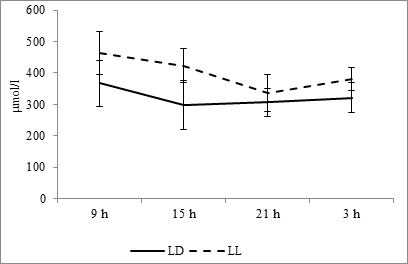
Fig. 5. Daily dynamics of uric acid in the blood plasma of rats at fixed light regime (LD) and constant illumination (LL).
The diurnal rhythmicity of another important metabolite – creatinine – both at the fixed light regime and at constant light are characterized by a maximum at 0900 hours and a minimum at 2100 hours (Graph 6). Cosinor analysis testifies that both at the fixed light regime and constant light an acrophase falls at morning hours – 0908 and 1011 hours, respectively, but in the second case, there is an increase in the rhythm amplitude from 2.32 µmol/l to 7.03 µmol/l (Fig. 10F).
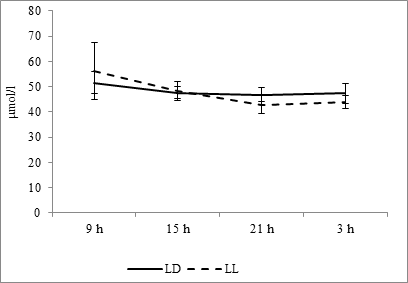
Fig. 6. Daily dynamics of creatinine in the blood plasma of rats in fixed light regime (LD) and constant illumination (LL).
The analysis of diurnal rhythmicity of aminotransferases allows us to reveal that the maximum contains of AST at both light regimes appears at 2100 hours and also as at fixed light regime and at the regime of constant light the minimum falls at 0300 hours (Fig. 7).
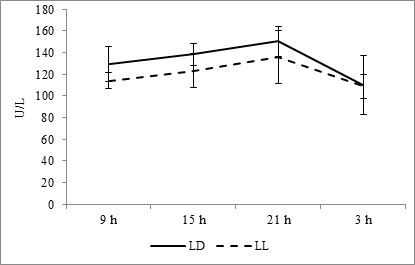
Fig. 7. Daily dynamics of AST in the blood plasma of rats at fixed light regime (LD) and constant illumination (LL).
The acrophase of rhythm at fixed light regime is noted at 1726 hours, at the regime of constant light it shifts at 2014 hours; the amplitude of rhythm is 17.41 U/L and 12.94 U/L, respectively (Fig. 10G).
In the case of ALT, the maximum is also noted at 1500 hours, minimum falls at evening and night hours – on 0300 at fixed light and 2100 hours at constant light regime (Fig. 8). At the same time, the diurnal dynamics does not have reliable circadian character (Fig. 10H).
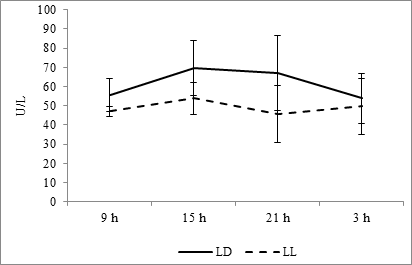
Fig. 8. Daily dynamics of ALT in the blood plasma of rats at fixed light regime (LD) and constant illumination (LL).
The activity of alkaline phosphatase at the fixed light regime is maximum at 2100 and comes to a minimum value at 0900 hours. At constant light regime, maximum shifts to 0300 hours and point of minimum do not change (Fig. 9).
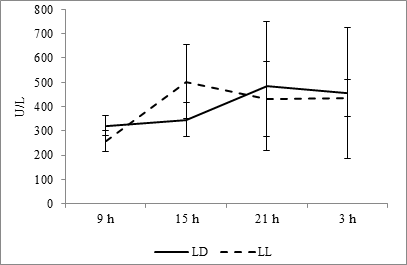
Fig. 9. Daily dynamics of alkaline phosphatase in the blood plasma of rats at fixed light regime (LD) and constant illumination (LL).
Cosinor analysis shows the absence of reliable CR both at the fixed light regime and at the regime of constant light (Fig. 10I).
 |
 |
|
A. |
B. |
 |
 |
|
C. |
D. |
 |
 |
|
E. |
F. |
 |
 |
|
G. |
H. |
 |
|
|
I. |
|
Fig. 10. Results of the cosinor analysis of circadian rhythm of rectal temperature (A), glucose (B), total protein (C), albumin (D), uric acid (E), creatinine (F), AST (G), ALT (H),and alkaline phosphatase (I) at fixed light regime (LD) and constant illumination (LL).
CONCLUSION
The conducted research testifies about the unambiguous modulating influence of constant illumination on the structure of diurnal dynamics of studied biochemical parameters and rectal temperature and about the initiation of desynchronosis.
The analysis of results allows noticing that under the influence of two-weeks-long constant lighting destruction of the circadian rhythm of the rectal temperature of male rats occurs.
A diurnal rhythm of body temperature is a combined result of changes in core temperature (internal organs) and skin temperature. It is known that the suprachiasmatic nuclei of the hypothalamus are a thermoregulatory center, signals from which initiate mechanisms of heat generation or loss, thus, creating a circadian rhythm of body temperature, which, in turn, can act as a regulator for other oscillators, for example, in regulation of the sleep-wake cycle [24-27]. At the same time, the circadian rhythm of temperature is one of the most stable.
It is well known that when the rhythms of illumination change, the endogenous rhythm of animals (including humans) starts to adapt to new external conditions. However, switching to new temporal pointers does not occur immediately. In humans, for example, such an adaptation can last from 1 to 3 weeks. In the process of restructuring of the temporal structure of an organism, the norms of daily curves, phases, periods, and amplitudes of fluctuations in the parameters of a person's health and his physiological processes start to change. At the same time, some of the directly diurnal rhythms restructure to the endogenous oscillator and become circadian (near-daily), while others continue to fluctuate in the diurnal regime. Thus, external desynchronization can trigger the occurrence of internal one [28]. It’s possible to assume that the restructuring of circadian rhythm is completed for 2 weeks.
At the same time, the restructuring of circadian rhythmicity of blood levels of glucose, creatinine, and AST was established.
It was not revealed reliable circadian rhythmicity for ALT, alkaline phosphatase and albumin both at the fixed light regime and in conditions of constant light, that allow suggesting the presence of a rhythm of higher or less continuance than circadian for the dynamics of these substances.
For the total protein and uric acid, no reliable circadian rhythms were observed at a fixed light regime, but its appearance was noted under constant illumination, which suggests that otherwise, regulators than the lighting rhythm may have a significant role in the regulation of daily rhythm.
Summarizing the above-mentioned, we can conclude that this study demonstrates new data on the rhythmicity of a number of physiological parameters of the mammalian organisms in normal conditions, as well as on the complex effect of the constant light regime on the functional state of an organism. The modulating effect of the lighting regime on the daily rhythm of a number of biochemical parameters of blood (glucose, creatinine, AST) and the destructive effect in case of the rhythm of temperature changes during the day are shown. Other parameters initially did not demonstrate the presence of daily rhythm (albumin, ALT, alkaline phosphatase). The occurrence of the daily rhythm of parameters that previously did not have it (total protein, uric acid) under the influence of constant illumination is especially remarkable.
The provided data can be used for further, more detailed study of the dynamics of physiological parameters under the influence of altered lighting conditions as is, and in combination with other stress factors.
REFERENCES
- Balsalobre A. Clock genes in mammalian peripheral tissues. Cell and tissue research. 2002 Jul 1;309(1):193-9.
- Boyce PR. Human factors in lighting. Crc Press; 2014 Apr 7.
- Foster RG, Roenneberg T. Human responses to the geophysical daily, annual and lunar cycles. Current biology. 2008 Sep 9;18(17):R784-94.
- Wehr TA. Photoperiodism in humans and other primates: evidence and implications. Journal of Biological Rhythms. 2001 Aug;16(4):348-6
- Hastings MH, Maywood ES, Brancaccio M. The mammalian circadian timing system and the suprachiasmatic nucleus as its pacemaker. Biology. 2019 Mar;8(1):13.
- Halberg F, Cornélissen G, Regal P, Otsuka K, Wang Z, Katinas GS, Siegelova J, Homolka P, Prikryl P, Chibisov SM, Holley DC. Chronoastrobiology: proposal, nine conferences, heliogeomagnetics, transyears, near-weeks, near-decades, phylogenetic and ontogenetic memories. Biomedicine & pharmacotherapy. 2004 Oct 1;58:S150-87.
- Halberg F. Chronobiology. Scholarpedia. 2011 Mar 17;6(3):3008.
- Rijo-Ferreira F, Takahashi JS. Genomics of circadian rhythms in health and disease. Genome Medicine. 2019 Dec;11(1):1-6.
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annual review of physiology. 1993 Mar;55(1):17-54.
- Gubin DG. Molecular basis of circadian rhythms and principles of circadian disruption. Uspekhi fiziologicheskikh nauk. 2013;44(4):65-87.
- Hozayen HM, Kamal HM, El-Gaidi MA, Mohamed A. Management of single metastatic tumor in the posterior fossa. Journal of Advanced Pharmacy Education & Research| Oct-Dec. 2017;7(4):410-3.
- Hardeland R, Madrid JA, Tan DX, Reiter RJ. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. Journal of pineal research. 2012 Mar;52(2):139-66.
- Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proceedings of the national academy of sciences. 2008 Sep 30;105(39):15172-7.
- Cao R. Molecular Biology and Physiology of Circadian Clocks. InOxford Research Encyclopedia of Neuroscience 2019 Jun 25.
- Stevens RG. Artificial lighting in the industrialized world: circadian disruption and breast cancer. Cancer Causes & Control. 2006 May 1;17(4):501-7.
- Moayeri A, Niazi H, Darvishi M. Effect of Biocanin A in the Acute Phase of Diffuse Traumatic Brain Injury . Int j pharm phytopharm res 2020;10(1):77-86.
- Shanafelt TD, Balch CM, Bechamps GJ, Shanafelt T, Bechamps G, Boone S, Tan L, Shirom A, Nirel N, Vinokur AD, Tilburt JC. 79 Shiftwork and Health. Cambridge Handbook of Psychology, Health and Medicine. 2019 Apr 30;11:357.
- Jasser SA, Blask DE, Brainard GC. Light during darkness and cancer: relationships in circadian photoreception and tumor biology. Cancer Causes & Control. 2006 May 1;17(4):515-23.
- Knutsson A. Health disorders of shift workers. Occupational medicine. 2003 Mar 1;53(2):103-8.
- Wang F, Zhang L, Zhang Y, Zhang B, He Y, Xie S, Li M, Miao X, Chan EY, Tang JL, Wong MC. Meta‐analysis on night shift work and risk of metabolic syndrome. Obesity reviews. 2014 Sep;15(9):709-
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proceedings of the National Academy of Sciences. 2010 Oct 26;107(43):18664-9.
- Vinogradova I, Anisimov V. Melatonin prevents the development of the metabolic syndrome in male rats exposed to different light/dark regimens. Biogerontology. 2013 Aug 1;14(4):401-9.
- Ashtiani AR, Vahidian-Rezazadeh M, Jafari M, Galdavi R, Mohammad M. Study of Changes in The Plasma Levels of Chemerin of Women with Overweight and Obese During a Period of Endurance Training On a Cycle-Ergometer Using Hydroalcoholic Extract of Urtica. Pharmacophore. 2018;9(2):72-9.
- Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. Journal of Neuroscience. 2006 Jun 14;26(24):6406-12.
- Refinetti R. The circadian rhythm of body temperature. Front Biosci. 2010 Jan 1;15(1):564-94.
- Scheer FA, Shea TJ, Hilton MF, Shea SA. An endogenous circadian rhythm in sleep inertia results in greatest cognitive impairment upon awakening during the biological night. Journal of biological rhythms. 2008 Aug;23(4):353-61.
- Basso A, Del Bello G, Piacenza F, Giacconi R, Costarelli L, Malavolta M. Circadian rhythms of body temperature and locomotor activity in aging BALB/c mice: early and late life span predictors. Biogerontology. 2016 Aug 1;17(4):703-14.
- Aschoff J. Biological Rhythms. Springer Science & Business Media, 2013.
