The Role of Fish Oil and Evening Primrose Oil against the Toxicity of Fenitrothion Pesticide in Male Rats
Aljadani, N.A.1*, Elnaggar, M.H.R.1,2, Assaggaff, A.I.1
1Department of Biological Sciences, Faculty of Sciences, King Abdulaziz University, Jeddah, KSA
2Department of Zoology, Faculty of Sciences, Suez Canal University, Ismailia, Egypt.
*Email: nuhaly @ gmail.com
ABSTRACT
Pollutants in the environment and exposure to them lead to medical ailments around the world. The present study aimed to evaluate the protective effect of evening primrose oil and fish oil against fenitrothion pesticide-induced toxicity in rats. Male Wistar rats weighing 150-220 g, were randomly distributed into seven groups, the first served as control. The second group received fenitrothion, the third and fourth groups were supplemented with fish oil and evening primrose oil, respectively. Rats of group five were exposed to fish oil and fenitrothion. The sixth group was exposed to evening primrose oil and fenitrothion. Rats of the seventh group were exposed to fish oil and evening primrose oil and fenitrothion. The results showed that the activities of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), and the levels of bilirubin (BIL), total protein (TP), albumin (ALB), glucose (GLU), cholesterol (CHOL), triglycerides (TG), low-density lipoprotein cholesterol (LDL-c), very low-density lipoproteins cholesterol (VLDL-c), and superoxide dismutase (SOD) were noticeably increased in rats administered fenitrothion. However, the level of serum high-density lipoprotein cholesterol (HDL-c) and glutathione (GSH) were markedly decreased. It was found that fish oil and evening primrose oil decreased the physiological unsettling influences initiated by fenitrothion. Moreover, the antioxidant properties of these oils supported the bioactive roles of its defensive impacts on fenitrothion toxicity. Finally, the present findings suggest that these oils may be utilized as preventive components against the toxicity of fenitrothion because of their antioxidant properties.
Key words: fenitrothion; fish oil; evening primrose oil; physiology, rats.
INTRODUCTION
Environmental pollution is the third issue of global crisis [1]. A wide range of chemicals is continuously released to the aquatic environments [2]. It can cause several prominent health issues. Pesticides are one of the renowned toxicity producers and cause environmental pollution [3]. There are many factors on which the toxicity of the pesticides relies upon for instance chemical properties, exposure period, doses, exposure methods, genetics, gender, age, the status of nutrition and variety of cases of individuals who suffer from several degrees of exposure [4].
The classification of pesticides was recorded based on the chemical structure. The World Health Organization (WHO) classified them, the spectrum of the family ranging from the organochlorine and organophosphorus compounds to inorganic compounds [5-7]. Organophosphorus compounds are one of the most common types of organic pollutants found in the environment [8]. Also, the organophosphorus pesticides can attack various systems of living bodies, for instance, nervous system, liver, immune system, urinary system and hematological system [9-20].
It was reported that organophosphorus pesticides cause oxidative stress and changes in the antioxidant system [21]. Whereas, the antioxidants provide a safeguard from the oxidative attacks in the body. There are several antioxidants reported in many scientific studies such as olive oil, corn oil, fish oil and evening primrose oil [4, 22, 23].
Fenitrothion (IUPAC name: O, O-dimethyl O-(3-methyl-4-nitrophenyl) phosphorothioate) is a pesticide classified in the family of organophosphate pesticides and comparatively inexpensive globally and thus widely used. It is one of the extensively used pesticides in agriculture [24]. Moreover, several investigations showed that fenitrothion could induce histopathological, biochemical and physiological alterations in experimental animals (El-Shenawy, 2010; Taib et al., 2013; Elzoghby et al., 2014; Abdel-Ghany et al., 2016; Jayusman et al., 2014; Tahoun et al., 2018) [21, 25-29].
Fish oil contains omega-3 polyunsaturated fatty acids (PUFAs) that appear to play several useful roles for human health [30]. Omega-3 (additionally alluded to as ω-3 or n-3) long-chain polyunsaturated fats are a group of mixes. There is growing evidence that fish oil may have great health benefits including reducing inflammation and the prevention of cancers [22, 31, 32].
Evening primrose (Oenothera biennis) is a plant belonging to family Onagraceae [33]. It contains about 50% to 70% linoleic acid (LA) and 7% to 10% γ-linolenic acid (GLA) [33, 34]. These essential fatty acids are important as cellular structural elements and as precursors of prostaglandins. Accordingly, evening primrose oil contains omega-6 acid, which may contribute to the proper functioning of human tissues because they are precursors of anti-inflammatory eicosanoids and decreased the seriousness of oxidative pressure [33, 35, 36].
The present study aimed to investigate the expected protective effect of evening primrose oil and fish oil against fenitrothion pesticide-induced biochemical and physiological alterations in male rats.
MATERIALS AND METHODS
Experimental Animals:
Adult male albino rats of the Wistar strain (Rattus norvegicus) weighing 150–220 g were used in the present study. Rats were housed in standard plastic cages and maintained under controlled laboratory conditions of humidity (65%), temperature (20±1°C) and 12:12 h light: dark cycle. Rats were fed on normal commercial chow and had free access to water ad libitum. The experimental treatments were conducted following the ethical guidelines of the Animal Care and Use Committee of King Abdulaziz University.
Experimental design:
A total of sixty-three adult male rats were randomly distributed into seven experimental groups, nine rats each and the experimental groups were treated as follows:
- Group 1: rats were untreated and served as control.
- Group 2: rats were orally administered ⅒LD50 dose of fenitrothion (30 mg/kg b.w.) by using the stomach tube day after day for four weeks.
- Group 3: rats were orally administered with 300 mg/kg b.w. of fish oil by using the stomach tube day after day for four weeks.
- Group 4: rats were orally administered with 300 mg/kg b.w. of evening primrose oil by using the stomach tube day after day for four weeks.
- Group 5: rats were orally administered with 300 mg/kg b.w. of fish oil then given ⅒LD50 dose of fenitrothion (30 mg/kg b.w.) using stomach tube day after day for four weeks.
- Group 6: rats were orally administered with 300 mg/kg b.w. of evening primrose oil and then given ⅒LD50 dose of fenitrothion (30 mg/kg b.w.) using stomach tube day after day for four weeks.
- Group 7: rats were orally administered with 300 mg/kg b.w. of evening primrose oil and 300 mg/kg b.w. of fish oil then given⅒LD50 dose of fenitrothion (30 mg/kg b.w.) using stomach tube day after day for four weeks.
Blood serum analyses:
At the end of the experimental period, rats fasted for 12 hours, water was not restricted and then anesthetized with diethyl ether. Blood samples were collected from orbital venous plexus in non-heparinized tubes, centrifuged at 2500 rpm for 15 minutes and blood sera were then collected and stored at -80°C. Serum levels of ALT and AST were determined using the methods of Reitman and Frankel (1957) [37]. The method of McComb and Bowers (1972) was carried out to determine the level of ALP [38]. The serum level of GGT was evaluated using the method of Szasz (1969) [39]. Serum levels of bilirubin, total protein, albumin, and glucose were determined using the methods of Doumas et al. (1973) [40], Peters (1968) [41], Doumas et al. (1997) and Trinder (1969) [42], respectively. The level of serum cholesterol was measured according to the method of Richmond (1973) [43]. The serum level of Triglycerides was estimated using the method of Fossati and Prencipe (1982) [44]. Serum levels of HDL-c, LDL-c, and VLDL-c were estimated using the method of Warnick (1983) and Friedewald et al. (1972), respectively [45, 46]. The method of Malstrom et al. (1975) was used to determine the level of serum SOD and the method of Beutler et al. (1963) was used to measure the level of serum GSH [47, 48].
Statistical Analysis:
The data were statistically analyzed with a completely randomized design by using the Statistical Package for Social Sciences (SPSS for Windows, version 12.0). Significant differences between treatments were tested with Student’s t-test and ANOVA analysis.
RESULTS
Levels of serum ALT, AST, ALP, GGT, bilirubin, total protein, albumin, glucose, cholesterol, triglycerides, HDL-c, LDL-c, VLDL-c, SOD and GSH in control, fenitrothion, fish oil, evening primrose oil, fish oil plus fenitrothion, evening primrose oil plus fenitrothion, fish oil and evening primrose oil mixture plus fenitrothion are shown in (Figs.1A-G). Fenitrothion administration showed a significant increase in the levels of serum ALT, AST, ALP, GGT, bilirubin, total protein, albumin, glucose, cholesterol, triglycerides, LDL-c, VLDL-c, and SOD; but the levels of serum HDL-c and GSH were decreased compared to control group.
Administration of fenitrothion resulted in a significant (P<0.01) increase in the activity of the enzymes ALT (311%), AST (403%), ALP (166%), and GGT (289%) compared to control group. Administration of fish oil and evening primrose oil resulted in nonsignificant increases in the activity of the enzymes. ALT (-1.8% and 0.2% respectively), AST (7.2% and -4.3% respectively), ALP (3.7% and -5.4% respectively) and GGT (-13.7% and 4.9% respectively) compared to control group. Moreover, treatment with fish oil and fenitrothion resulted in significant (P<0.01) decreases in the levels of ALT (-54.17%), AST (-54.70%), ALP (-41.40%) and GGT (-50.88%) compared to fenitrothion group. Also, there were significant (P<0.01) decreases in the levels of ALT (-64.72%), AST (61.73%), ALP (-57.10%) and GGT (-53.22%) in rats treated with both evening primrose oil and fenitrothion compared to fenitrothion group. Moreover, the levels of ALT (-77.86%), AST (-80.78%), ALP (-65.36%) and GGT (-69.79%) were significantly (P<0.01) decreased in rats treated fish oil and evening primrose oil and fenitrothion when compared to fenitrothion group (Table 1).
Administration of fenitrothion G2 resulted in significant (P<0.01) increases in serum bilirubin concentration (213%), total protein (34%), albumin (83.9%) and glucose (86.9%) compared to control group. Administration of fish oil G3 and evening primrose oil G4 resulted in nonsignificant increases in the levels of serum bilirubin (0% and -8.1% respectively), total protein (-0.4% and 3.5% respectively), albumin (6.6% and 0.4% respectively) and glucose (7.4% and -2.5% respectively) compared to control group. Administration of fish oil and fenitrothion resulted in significant (P<0.01) decreases in the levels of serum bilirubin (-64.40%), total protein (-21.25%), albumin (-45.63%) and glucose (-38.51%) compared to fenitrothion group. Administration of evening primrose oil and fenitrothion resulted in significant (P<0.01) decreases in the levels of serum bilirubin (-71.73%), total protein (-19.77%), albumin (-43.52%) and glucose (-51.07%) compared to fenitrothion group.
Administration of fish oil with evening primrose oil plus fenitrothion resulted in significant (P<0.01) decreases in the levels of serum bilirubin (-78.01%), total protein (-28.22%), albumin (-45.26%) and glucose (-51.07%) compared to fenitrothion group (Table 2).
Administration of fenitrothion G2 resulted in significant (P<0.01) increases in the concentration of the serum cholesterol (79.1%), triglyceride (78%), LDL (134%) and VLDL (77.9%) compared to control group. On the other hand, the administration of fenitrothion G2 resulted in significant (P<0.01) decreases in serum HDL concentration (-23%) compared to the control group. Administration of fish oil G3 and evening primrose oil G4 resulted in non significant changes in the concentrations of the serum cholesterol (-1.5% and 22.1% respectively), triglyceride (0.2% and -0.5% respectively), HDL (0.8% and 1.1% respectively), LDL (-2.8% and 33.4% respectively), and VLDL (0.1% and 0.5% respectively) compared to control group. Administration of fish oil with fenitrothion resulted in significant (P<0.01) decreases in serum cholesterol concentrations (-39.80%), triglyceride (-45.31%), LDL (-52.77%), and VLDL (-45.30%) compared to fenitrothion group. On the other hand, the administration of fish oil with fenitrothion resulted in significant (P<0.01) increases in serum HDL concentrations (34.32%) compared to the fenitrothion group. Administration of primrose oil with fenitrothion resulted in significant (P<0.01) decreases in the concentrations of the serum cholesterol (-45.38%), triglyceride (-44.44%), LDL (-60.70%), and VLDL (-44.44%) compared to fenitrothion group. On the other hand, the administration of primrose oil plus fenitrothion resulted in significant (P<0.01) increases in serum HDL concentrations (42.09%) compared to the fenitrothion group. Administration of fish oil with evening primrose oil plus fenitrothion resulted in significant (P<0.01) decreases in the concentrations of the serum cholesterol (-44.02%), triglyceride (-39.58%), LDL (-59.08%) and VLDL (-39.59%) compared to fenitrothion group. On the other hand, the administration of fish oil plus evening primrose oil with fenitrothion resulted in significant (P<0.01) increases in the concentrations of the serum HDL (42%) compared to fenitrothion group (Table 3).
Administration of fenitrothion G2 resulted in significant (P<0.01) increases in the concentration of the serum SOD (66.2%) compared to the control group. On the other hand, the administration of fenitrothion G2 resulted in significant (P<0.01) decreases in serum GSH concentration (-80.3%) compared to the control group. Administration of fish oil G3 with evening primrose oil G4 resulted in no significant changes in serum SOD concentrations (-1.9% and -0.0% respectively) and GSH (-5.4% and 5.3% respectively) compared to the control group. Administration of fish oil with fenitrothion resulted in significant (P<0.01) decreases in serum SOD concentrations (-36.19%) compared to the fenitrothion group. On the other hand, the administration of fish oil with fenitrothion resulted in a significant (P<0.01) increase in serum GSH concentrations (436.88%) compared to the fenitrothion group. Administration of primrose oil with fenitrothion resulted in significant (P<0.01) decreases in serum SOD concentrations (-34.65%) compared to the fenitrothion group. On the other hand, the administration of primrose oil plus fenitrothion resulted in significant (P<0.01) increases in serum GSH concentrations (379.88%) compared to the fenitrothion group. Administration of fish oil plus evening primrose oil with fenitrothion resulted in significant (P<0.01) decreases in the concentrations of the serum SOD (-42.98%) compared to fenitrothion group. On the other hand, the administration of fish oil plus evening primrose oil with fenitrothion resulted in significant (P<0.01) increases in the concentrations of the serum GSH (414.87%) compared to fenitrothion group (Table 4).
Table 1: Effect of fish oil, primrose oil, and their combination on serum ALT, AST, ALP, and GGT levels measured in control and fenitrothion-treated animals.
|
Parameter Groups |
ALT (U/L) |
AST (U/L) |
ALP (U/L) |
GGT (U/L) |
|
Control (G1) |
17.48±3.76 |
20.97±7.43 |
51.00±6.87 |
19.45±3.28 |
|
Fenitrothion (G2) |
72.00±11.75a (311%) |
105.58±8.83a (403%) |
135.67±13.31a (166) |
75.67±13.63a (289%) |
|
Fish Oil (G3) |
17.16±3.28b (-1.8%) |
22.50±6.40b (7.2%) |
52.89±6.53b (3.7%) |
16.78±3.23b (-13.7%) |
|
Primrose Oil (G4) |
17.52±3.10b (0.2%) |
20.06±4.14b (-4.3%) |
48.22±4.74b (-5.4%) |
20.42±5.86b (4.9%) |
|
Fish Oil + Fenitrothion (G5) |
33.00±7.46a,b,c (88.7%) |
47.83±4.62a,b,c (128%) |
79.50±14.24a,b,c (55.8%) |
37.17±7.03a,b,c (91.1%) |
|
% differencefrom (G2) |
-54.17% |
-54.70% |
-41.40% |
-50.88% |
|
Primrose Oil + Fenitrothion (G6) |
25.40±5.94a,b,d (45.3%) |
40.40±2.70a,b,d (92.6%) |
58.20±13.41b (14.1%) |
35.40±8.65a,b,d (82%) |
|
% differencefrom (G2) |
-64.72% |
-61.73% |
-57.10% |
-53.22% |
|
Fish Oil + Primrose Oil + Fenitrothion (G7) |
15.94±2.44b (-0.8%) |
20.29±2.14b (-3.2%) |
47.00±2.83b,c (-7.8%) |
22.86±6.44b,c (17.5%) |
|
% difference from (G2) |
-77.86% |
-80.78% |
-65.36% |
-69.79% |
Results are expressed as mean ± SEM. a P<0.01 significant compared to the control group. b P<0.01 significant compared to the fenitrothion group. cP<0.01 significant compared to the fish oil + fenitrothion group. d P<0.01 significant compared to the primrose oil + fenitrothion group. ( ) Percentage difference from control
Table 2: Effect of fish oil, primrose oil, and their combination on serum bilirubin, total protein, albumin, and glucose levels measured in control and fenitrothion-treated animals.
|
Parameter Groups |
Bilirubin(mg\dl) |
Total Protein (g\dl) |
Albumin (g\dl) |
Glucose (mg\dl) |
|
Control G1 |
0.61±0.25 |
7.01±0.74 |
4.36±0.65 |
94.83±6.68 |
|
Fenitrothion G2 |
1.91±0.15a (213%) |
9.46±0.44a (34%) |
8.02±0.88a (83.9%) |
177.33±16.61a (86.9%) |
|
Fish Oil G3 |
0.61±0.22b (0%) |
6.98±0.71b (-0.4%) |
4.65±0.71b (6.6%) |
101.89±9.13b (7.4%) |
|
Primrose Oil G4 |
0.56±0.21b (-8.1%) |
7.26±0.84b (3.5%) |
4.38±0.61b (0.4%) |
92.44±14.94b (-2.5%) |
|
Fish Oil + Fenitrothion G5 |
0.68±0.24b (11.4%) |
7.45±0.48b (6.2%) |
4.36±0.47b (0%) |
108.83±18.10b (14.7%) |
|
% difference from (G2) |
64.40%- |
-21.25% |
45.63%- |
-38.51% |
|
Primrose Oil + Fenitrothion G6 |
0.54±0.22b (-11.4%) |
7.59±0.66b (8.2%) |
4.53±0.55b (3.8%) |
86.60±13.59b (-8.6%) |
|
% difference from (G2) |
71.73%- |
-19.77% |
43.52%- |
-51.07% |
|
Fish Oil + Primrose Oil + Fenitrothion G7 |
0.42±0.27b (-31.4%) |
6.79±0.62b (-3.1%) |
4.39±0.64b (0.6%) |
86.00±7.85b,c (-9.3%) |
|
% difference from (G2) |
78.01%- |
-28.22% |
45.26%- |
-51.41% |
Results are expressed as mean ± SEM. a P<0.01 significant compared to the control group. b P<0.01 significant compared to the fenitrothion group. cP<0.01 significant compared to the fish oil + fenitrothion group. ( ) Percentage difference from control.
Table 3: Effect of fish oil, primrose oil, and their combination on serum cholesterol, triglyceride, high-density lipoproteins (HDL), low-density lipoproteins (LDL), and very-low-density lipoproteins (VLDL) levels measured in control and fenitrothion-treated animals.
|
Parameter Groups |
Cholesterol (mg/dl) |
Triglyceride (mg/dl) |
HDL (mg/dl) |
LDL (mg/dl) |
VLDL (mg/dl) |
|
Control G1 |
125.50 ±13.59 |
75.83 ±7.78 |
43.83 ±3.49 |
81.67 ±13.72 |
15.17 ±1.56 |
|
Fenitrothion G2 |
224.83 ±66.34a (79.1%) |
135.00 ±53.09a (78%) |
33.50 ±8.17a (-23%) |
191.33 ±73.49a (134%) |
27.00 ±10.62a (77.9%) |
|
Fish Oil G3 |
123.56 ±12.34b (-1.5%) |
76.00 ±4.39b (0.2%) |
44.22 ±4.99b (0.8%) |
79.33 ±13.42b (-2.8%) |
15.20 ±0.88b (0.1%) |
|
Primrose Oil G4 |
153.33 ±14.82a,b (22.1%) |
75.44 ±6.23b (-0.5%) |
44.33 ±4.03b (1.1%) |
109.00 ±14.60a,b (33.4%) |
15.09 ±1.25b (-0.5%) |
|
Fish Oil + Fenitrothion G5 |
135.33 ±14.46b (7.8%) |
73.83 ±4.45b (-2.6%) |
45.00 ±4.34b (2.6%) |
90.33 ±12.99b (10.6%) |
14.77 ±0.89b (-2.6%) |
|
% difference from (G2) |
-39.80% |
-45.31% |
34.32% |
-52.77% |
-45.30% |
|
Primrose Oil + Fenitrothion G6 |
122.80 ±11.08b,d (-2.1%) |
75.00 ±4.18b (-1.0%) |
47.60 ±6.88b (8.6%) |
75.20 ±8.79b,d (-7.9%) |
15.00 ±0.84b (-1.1%) |
|
% difference from (G2) |
-45.38% |
-44.44% |
42.09% |
-60.70% |
-44.44% |
|
Fish Oil + Primrose Oil + Fenitrothion G7 |
125.86 ±11.42b,d (0.2%) |
81.57 ±9.88b (7.5%) |
47.57 ±4.12b (8.5%) |
78.29 ±11.91b,d (-4.1%) |
16.31 ±1.98b (7.5%) |
|
% difference from (G2) |
-44.02% |
-39.58% |
42% |
-59.08% |
-39.59% |
Results are expressed as mean ± SEM. a P<0.01 significant compared to the control group. b P<0.01 significant compared to the fenitrothion group. ( ) Percentage difference from control.
Table 4: Effect of fish oil, primrose oil, and their combination on serum superoxide dismutase (SOD) and reduced glutathione (GSH) levels measured in control and fenitrothion-treated animals.
|
Parameter Groups |
SOD (µ\ml) |
GSH (ng\ml) |
|
Control G1 |
181.17±12.73 |
17.47±2.48 |
|
Fenitrothion G2 |
301.17±11.21a (66.2%) |
3.43±1.14a (-80.3%) |
|
Fish Oil G3 |
177.67±10.33b (-1.9%) |
16.52±2 (-5.4%) |
|
Primrose Oil G4 |
181.00±12.18b (-0.0%) |
18.41±2.65b (5.3%) |
|
Fish Oil + Fenitrothion G5 |
192.17±23.13b (6.07%) |
18.82±2.74b (7.7%) |
|
% difference from (G2) |
-36.19% |
436.88% |
|
Primrose Oil + Fenitrothion G6 |
196.80±5.07b,d (8.6%) |
16.46±2.26b (-5.7%) |
|
% difference from (G2) |
-34.65% |
379.88% |
|
Fish Oil + Primrose Oil + Fenitrothion G7 |
171.71±6.52b,d (-5.2%) |
17.66±2.82b (1.0%) |
|
% difference from (G2) |
-42.98% |
414.87% |
Results are expressed as mean ± SEM. a P<0.01 significant compared to the control group. b P<0.01 significant compared to the fenitrothion group. c P<0.05 significant compared to the primrose oil + fenitrothion group. ( ) Percentage difference from control.
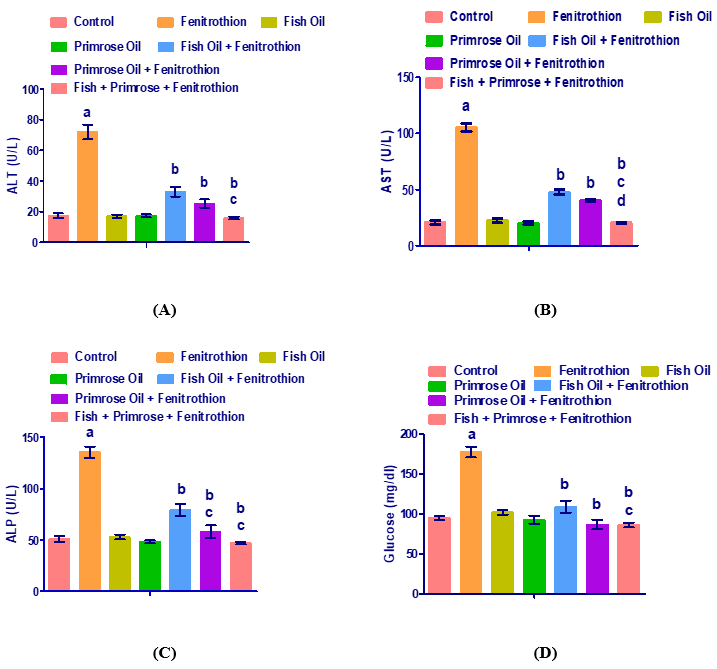
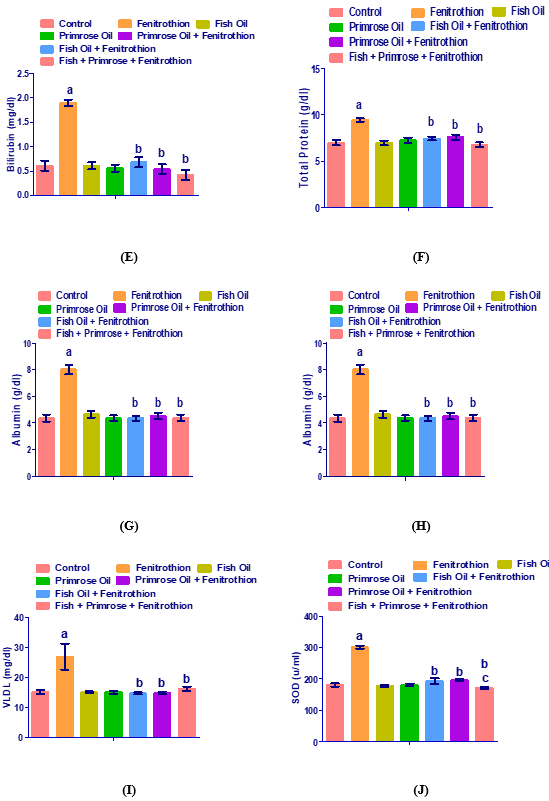
Figure 1. Levels of serum ALT (A), AST (B), ALP (C), GGT (D), bilirubin (E), total protein (F), albumin (G), glucose (H), cholesterol (I), triglycerides (J), HDL (O), LDL (L), VLDL (M), SOD (N) and GSH (K) in serum from control, fenitrothion, fish oil, evening primrose oil, fish oil plus fenitrothion, evening primrose oil plus fenitrothion and fish oil with evening primrose oil plus fenitrothion-treated rats.
DISCUSSION
The exposure of humans to pesticides and other toxicants has turned to a chief health issue. Organophosphorus insecticides have been linked with numerous physiological, biochemical and histopathological modifications [4, 49, 50]. The present study was aimed to evaluate whether pretreatment with fish oil and evening primrose oil would have protective effects on fenitrothion-induced hepatorenal injury in male rats. In this study, levels of serum ALT, AST, ALP, GGT, bilirubin, total protein, albumin, glucose, cholesterol, triglycerides, LDL-c, VLDL-c, and SOD were significantly higher in fenitrothion-treated rats. While there were decreases in the level of serum HDL and GSH in rats exposed to fenitrothion. ALP is a zinc metalloenzyme, which has a centered serine moiety; they liberate inorganic phosphate from various organic orthophosphates. The enzyme is present in almost all body tissues. In the liver, ALP is present in the bile ducts microvilli and on the surface of the hepatocytes. The increased serum concentration of ALP is usually presented during liver illness or bone disease [51].
AST and ALT enzymes are found normally in the cytosol of hepatocytes and have a role in converting amino acids into alpha-keto acids. In cases of hepatotoxicity, where the cell loses the protective membrane, the enzymes leak into the blood and their levels rise. Therefore, AST and ALT are used to examine liver function. There is an inverse relationship between the level of AST and ALT and the liver function [52-54].
GGT is a glycoprotein attached to the cell membrane. GGT supports the transport of the gamma-glutamyl group to peptides, amino acids, and water. The level of GGT increases markedly in the case of liver diseases such as acute viral hepatitis and cholestasis. It is used to differentiate if the elevated level of ALP is due to liver or bone disease because in the case of hepatic damage GGT correlates well with ALP [51].
Exposure to organophosphate pesticide is the main cause of elevated serum ALT, AST, ALP, and GGT levels which were previously reported in several studies [4, 12, 55-59]. In the present study, fenitrothion illustration noticeably increased ALP, AST, ALP and GGT activity as compared to the control group. This result agrees with many previous findings [21, 25, 26, 28, 60, 61].
Furthermore, in the present study, fenitrothion administration significantly influenced the excretory and synthetic function of the liver as indicated by the observed alterations that occurred in albumin, bilirubin, total protein, glucose, triglycerides, cholesterol, HDL-c, LDL-c, and VLDL-c. The research of Abdel-Ghany et al. (2016) supported this study's results. The study examined the influence of oral administration fenitrothion on the liver, lung function and kidney of rats. The findings of the research illustrated that the damage in the functioning of the liver was reported, which can be validated by the increase in the bilirubin level. Moreover, like this study results, Elhalwagy et al. (2008) research found that fenitrothion caused an increase of total cholesterol, triglycerides and glucose levels while causes a decrease in plasma total protein [62]. The results of the present study agreed also with those obtained by Zidan (2015) who declared an increase in albumin, protein, bilirubin, and cholesterol in animals treated with pesticides. These study results are consistent with the data reported by Al-attar et al. (2017) which showed that the levels of total protein, albumin, glucose, and total cholesterol were markedly elevated in rats administered with diazinon. Opposite to our results are those of Afshar et al. (2008) who found a decrease in total protein and triglycerides levels in rats treated with fenitrothion [63].
During the present work, a marked elevation in the concentration of triglycerides and cholesterol in the serum of rats exposed to fenitrothion, a remarkable decline in the level of HDL-c, and a remarkable increase in the concentration of LDL-c were observed. The current alterations in lipids picture coincide with prior investigations that concluded that the animals supplied with insecticides exhibited serious changes in concentrations of different blood lipids [12, 13, 64-69]. Increased serum triglyceride levels together with a reduced level of serum HDL-c comprise one of the most prevalent atherogenic pictures of lipid metabolism. These modifications in the blood lipid picture may be referred to the stimulated lipolysis and fatty acid synthesis. The expansion in serum all out cholesterol levels may be attributed to the blockage of the liver bile canaliculus, which lessens or stops cholesterol emission into the duodenum [70]. Expanded serum cholesterol levels might be an indication of liver harm. Besides that, Agbor et al. (2005) told that the expansion in blood triglycerides levels may result from an increment in the pace of arrangement and the pace of discharge of triglycerides by the parenchyma cells into the circulation system [71]. Moreover, the expansion in serum cholesterol might be ascribed with the impact of pesticides on the penetrability of the liver cell membrane [72].
It is obvious from the present results that, the concentration of serum glucose displayed a significant increase in rats administered fenitrothion. Hyperglycemia was reported as one of the famous symptoms of insecticides’ toxicity by Lasram et al. (2009) and Ruckmani et al. (2011) [73, 74]. Thus, blood glucose might be used as an indicator of the toxicity of organophosphorus insecticides. Both increase in glucose production and a decrease in glucose utilization may lead to hyperglycemia [75]. Besides, Pournourmohammadi et al. (2007) disclosed that the clear alteration in serum sugar concentration after insecticides exposure is suggestive of altered carbohydrate metabolism and increases the hydrolysis of liver glycogen [76].
Reduced glutathione (GSH) played an important role in antioxidant defense. It might directly scavenge free radicals or indirectly converted free radicals by glutathione S-transferase and glutathione peroxidase to a non-toxic species [77]. This study data showed that fenitrothion significantly decreased GSH activity compared to normal control levels. Supporting the present study results, were those of Ahmed et al. (2000) who demonstrated that, subchronic exposure to malathion caused the increase in the malondialdehyde concentration [78], GSH concentration, and decreases in the activities of superoxide dismutase (SOD), catalase, glutathione peroxidase, glutathione reductase and glutathione-S-transferase in the RBC of rats. They suggested that organophosphorus insecticides might incite oxidative pressure prompting of free radicals and modifications non-enzymatic and enzymatic antioxidants. The antioxidant enzymatic system constitutes the main internal mechanism that is responsible for the eradication of the free radicals produced in the body. SOD save tissues from superoxide radical [79]. This study data showed that fenitrothion significantly increased SOD activity compared to normal control levels. Opposite to this study results, previous works showed reduced SOD activity following fenitrothion injection in rats, indicating deteriorated antioxidant activity during massive free radicals’ production which is usually removed by the antioxidant enzymes such as SOD [80, 81]. The results of the present study agreed with many other studies’ findings [4, 82, 83]. El-Shenawy (2010) showed that fenitrothion, endosulfan, and abamectin decreased the activities of the antioxidant enzyme glutathione peroxidase mostly by fenitrothion.
There are plentiful kinds of omega-3 fatty acids that vary depending on their chemical structure and molecular size. The three most known omega-3 unsaturated fats are Eicosapentaenoic acid (EPA), Docosahexaenoic acid (DHA) and alpha-linolenic acid (ALA). EPA primary capacity is to deliver chemicals called Eicosanoids, which help reduce inflammation [31]. DHA is an exceedingly substantial factor for healthy brain growth and function [84]. ALA can be converted into EPA and DHA. ALA is one of the fundamental sources of energy production in living organisms [85].
Fish oil is the oil extracted from the tissues of oily fish. They constitute the omega-3 fatty acids, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). These unsaturated fats are antecedents of specific eicosanoids that are perceived to limit irritation in the body and improve hypertriglyceridemia [85-87]. The most promising evidence supports supplementation for the prevention of cardiac death [88]. Furthermore, fish oil and omega-3 fatty acids have been investigated in a wide range of other diseases like depression [89, 90], anxiety [91-93], tumors, and macular degeneration, and so far their advantageous role in these diseases has also not been proved.
Evening primrose oil contains about 50% to 70% cis-linoleic acid (LA) and 7% to 10% cis-gamma (GLA) [33, 94]. These essential unsaturated fats are significant as cell basic components and as antecedents of prostaglandins.
In this study, fish oil and primrose oil, natural products were used that are rich in omega-3 fatty acids, the prominent antioxidant molecules. The results demonstrated a decrease in the toxic effects of fenitrothion on the liver synthetic and excretory function and an increase in the non-enzymatic antioxidant, GSH in fish oil, primrose oil, and the mixture groups. Omega-3 fats are a crucial part of human cell membranes. It helps decrease the number of liver fats [95]. Spadaro et al. (2008) reported that serum ALT and triglyceride levels, as well as fatty liver, improved after polyunsaturated fatty acid administration. Besides, fish oil reversed high-fructose diet-induced dyslipidemia, as it significantly decreased triglycerides level [96]. A recent meta-analysis has reported that omega-3 fatty acids improved liver fat, GGT, triglyceride, and HDL in patients with non-alcoholic fatty liver disease [97]. Moreover, omega-3 fatty acids significantly decreased diclofenac-induced liver injury as it significantly decreased the activities of ALT, AST, and alkaline phosphatase, but, significantly raise the total antioxidant capacity and the activities of superoxide dismutase, catalase, and glutathione peroxidase [98]. The results of Moghadamnia et al. (2017) showed that the administration of fish oil omega-3 product produced a hepatoprotective impact against thioacetamide model of liver toxicity [99]. They showed that the mean concentration of ALT, AST, GGT, LDH, and bilirubin exhibited a pronounced reduction in the animals supplemented with fish oil omega-3 rather than thioacetamide supplemented group. Furthermore, the fish oil omega-3 treated group offered a marked lowered serum albumin level.
It was presented that omega-3 fatty acid exerted a favorable impact on the antioxidant status of both healthy animals as well as animals’ model of oxidative stress [100-102]. Postnatal consumption of omega-3-containing food repaired prenatal ethanol-induced oxidative stress as confirmed by increased GSH and decreased thiobarbituric acid reactive substances [100]. Martorell et al. (2015) reported that athletes who administered diet mixed with DHA and have a 25% increase in serum concentration of DHA ameliorated serum SOD and CAT and reduced MDA and nitrotyrosine [103].
The results of other authors agree with the present study results, as El-Kossi et al. (2011) stated that the impact of treatment with evening primrose oil in diabetic rats significantly decreased serum levels of glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol, thiobarbituric reactive substance (a marker of oxidative pressure), and increased the levels of high-density lipoprotein cholesterol and total antioxidant capacity.
The results obtained in this study agreed with the results of many previous works. It appears that the consumption of fish oil omega-3 complements preserved against the fenitrothion model of hepatotoxicity by quenching reactive oxygen species and enhancement of GSH synthesis. Because there are no previous studies that describe the hepatoprotective action of fish oil omega-3 complements in the fenitrothion model of liver damage, it is impossible to compare this work’s results with others. Accordingly, further experimental works should proceed in order to investigate the liver antioxidant enzymes and molecular alterations stimulating apoptosis, hence the impacts of fish oil omega-3 complements on curing liver damage can be estimated with a lot of reality. The result of this work presented that both fish oil and primrose oil improved liver function in this mice model of hepatotoxicity. Consequently, if confirmed by more studies, it is likely to combine fish oil omega-3 complements and/or primrose oil with the food of persons with fenitrothion-induced hepatotoxicity.
The results of the present study could conclude that consumption of fish oil and/or primrose oil protected the rats against the deleterious effects of fenitrothion organophosphorus insecticides. In addition, fish oil and/or primrose oil protected the liver and testis against the damaging effect of fenitrothion. Fish oil and/or primrose oil also exerted an anti-hyperlipidemic effect in fenitrothion-induced toxicities. These actions of fish oil and/or primrose oil may result from their antioxidant effects.
REFERENCES
- Feghhi F, Malekzadeh M. Nurses’ Attitude Towards the Care of Narcotic Patients in Yasuj in 2014. Int. J. Pharm. Phytopharm. Res. 2018; 8(3): 55-58.
- Al-Ansari A M. Steroidal estrogens occurrence and removal in two STPs and coastal marine water from jeddah, saudi arabia. Pharmacophores. 2018; 9(1): 69-79.
- Al-Maathidy A, Alzyoud J A M, Al-Dalaen S, Al-Qtaitat A. Histological alterations in the Thyroid Follicular cells induced by lead acetate toxicity in adult male albino rats. Int. j. pharm. phytopharm. Res. 2019; 9(5): 19-26.
- Al-Attar, A.M., Elnaggar, M.H., Almalki, E.A. Protective effect of some plant oils on diazinon induced hepatorenal toxicity in male rats. Saudi J. Biol. Sci., 2017 ; 24(6), 1162-1171.
- World Health Organization (WHO).The WHO recommended classification of pesticides by hazard and guidelines to classification: 2004, World Health Organization, Ginebra, 2004.
- World Health Organization (WHO). The WHO specification and evaluation for public health pesticides fenitrothion: 2009, World Health Organization, 2009.
- Garcia, F.P., Ascencio, S.Y.C., Oyarzun, J.G., Hernandez, A.C., Alavarado, P.V. Pesticides: Classification, uses and toxicity. Measures of exposure and genotoxic risks. J. Res. Environ. Sci. Toxicol., 2012 ; 1(11), 279-293.
- Tang, J., Zhang, M., Cheng, G., Lu, Y. Diazinon determination using high performance liquid chromatography: a comparison of the ENVI-Carbcolumnwith the immunoaffinity column for the pretreatment of water and soil samples. Bull. Environ. Cont. Toxicol., 2009 ; 83(5), 626-629.
- Benjamin, N., Kushwah, A., Sharma, R. K., Katiyar, A. K. Histopathological changes in liver, kidney and muscles of pesticides exposed malnourished and diabetic rats. CSIR, 2006 ; 44(3): 228-232.
- Al-Attar, A.M. The ameliorative role of β-carotene pretreatment on diazinon-induced enzymological and histopathological changes in Wistar male rats. Glob. J. Pharmacol., 2009 ; 3(3), 171-177.
- Al-Attar, A.M. Physiological and histopathological investigations on the effects of 𝛼-lipoic acid in rats exposed to malathion. BioMedRes. Int., 2010.
- Al-Attar, A.M. Effect of grape seed oil on diazinon-induced physiological and histopathological alterations in rats. Saudi J. Biol. Sci., 2015 ; 22(3), 284-292.
- Al-Attar, A.M., Abu Zeid, I.M. Effect of tea (Camellia sinensis) and olive (Olea europaea L.) leaves extracts on male mice exposed to diazinon. BioMed. Res. Int., vol. 2013, 461415, Doi: 10.1155/2013/461415
- Al-Attar, A.M., Al-Taisan, W.A.A. Preventive effects of black seed (Nigella sativa) extract on Sprague Dawley rats exposed to diazinon. Aust. J. Basic Appl. Sci., 2010 ; 4(5), 957-968.
- Holy, B., Kenanagha, B., Onwuli, D.O. Haemato-pathological effect of dichlorvos on blood picture and liver cells of albino rats. J. Toxicol. Environ. HealthSci., 2015 ; 7(2), 18-23.
- Tian, J., Dai, H., Deng, Y., Zhang, J., Li, Y., Zhou, J., Zhao, M., Zhao, M., Zhang, C., Zhang, Y., Wang, P. Bing, G., Zhao, L. The effect of HMGB1 on sub-toxic chlorpyrifos exposure-induced neuroinflammation in amygdala of neonatal rats. Toxicol., 2015 ; 338, 95-103.
- Abdel-Daim, M.M. Synergistic protective role of ceftriaxone and ascorbic acid against subacute diazinon-induced nephrotoxicity in rats. Cytotechnol., 62016 ; 8(2), 279-289.
- Judge, S. J., Savy, C. Y., Campbell, M., Dodds, R., Gomes, L. K., Laws, G., Watson, A., Blain, P., Morris, C., Gartside, S. E. Mechanism for the acute effects of organophosphate pesticides on the adult 5-HT system. Chemico-Biolog. Interact., 2016 ; 245, 82-89.
- Li, S., Cao, C., Shi, H., Yang, S., Qi, L., Zhao, X., Sun, C. Effect of quercetin against mixture of four organophosphate pesticides induced nephrotoxicity in rats. Xenobiotica, 2016 ; 46(3), 225-233.
- Mehri, N., Felehgari, H., Harchegani, A.L., Behrooj, H., Kheiripour, N., Ghasemi, H., Mirhoseini, M., Ranjbar, A. Hepatoprotective effect of the root extract of green tea against malathion induced oxidative stress in rats. J. HerbMed. Pharmacol. 2016 ; 5, 116–119.
- Abdel-Ghany, R., Mohammed, E., Anis, S., Barakat, W. Impact of exposure to fenitrothion on vital organs in rats. J. Toxicol., 2016, 2016: 5609734. doi:10.1155/2016/5609734.
- Nunes, M. L., Schemitt, E., Porawski, M, Anti-inflammatory and antioxidant effect of long chain omega-3 polyunsaturated fatty acids (LC n-3 PUFAs) in diabetic rats. Inter. J. Food Sci. Nutr., 2018 ; 3(5). 25-32.
- Mikešová, K., Härtlová, H., Zita, L., Chmelíková, E., Hůlková, M., Rajmon, R. Effect of eveningprimroseoilevening primrose oil on biochemical parameters of thorough bred horses under maximal training conditions. Czech J. Anim. Sci., 2014 ; 59(10), 488-493.
- Saber, T. M., Abd El‐Aziz, R. M., Ali, H. A, Quercetin mitigates fenitrothion‐induced testicular toxicity in rats. Andrologia, 2016 ; 48(5), 491-500.
- El-Shenawy, N.S. Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytes. Toxicol. in vitro, 2010 ; 24(4), 1148-1157.
- Taib, I. S., Budin, S. B., Ghazali, A. R., Jayusman, P. A., Louis, S. R., Mohamed, J. Fenitrothion induced oxidative stress and morphological alterations of sperm and testes in male spragueSprague-dawley Dawley rats. Clinics, 2013 ;68(1), 93-100.
- Elzoghby, R.R., Ahlam, F.H., Abdel-Fatah, A., Farouk, M. Protective role of vitamin C and green tea extract on malathion-induced hepatotoxicity and nephrotoxicity in rats. . Am. J. Pharmacol. Toxicol., 2014 ; 9(3), 177.
- Jayusman, P.A., Budin, S.B., Ghazali, A.R., Taib, I.S., Louis, S.R. Effects of palm oil tocotrienol-rich fraction on biochemical and morphological alterations of liver in fenitrothion-treated rats. Pak. J. Pharm. Sci., 2014 ; 27(6), 1873-1880.
- Tahoun, E.A., Mohammed, R.S., Donia, G.R. Histopathological and Biochemical Studies on The Effect of Green Tea Extract and vitamin C Against Fenitrothion Toxicity in Male Albino Rats. Alex. J. Vet. Sci., 2018 ; 57(1), 68-80.
- Sidhu, K.S. Health benefits and potential risks related to consumption of fish or fishoilfish oil. Reg. Toxicol. Pharmacol., 2003 ; 38(3), 336-344.
- Calder, P.C.. Omega-3 fatty acids and inflammatory processes. Nutrients, 2010 ; 2(3), 355-374.
- Kazuo, M. Prevention of Fish Oil Oxidation. J. OleoSci., 2019 ; 68(1), 1-11.
- Timoszuk, M., Bielawska, K., Skrzydlewska, E.Evening primrose (Oenothera biennis) biological activity dependent on chemical composition. Antioxidants, 2018 ; 7(8), 108.
- Hudson, B.J.F. Evening primrose (Oenothera spp.) oil and seed. J. Am. Oil Chem. Soc.; 1984 ; 61: 540–543.
- Kaya, Z., Eraslan, G. The effects of eveningprimroseoilevening primrose oil on arsenic-induced oxidative stress in rats. Toxicol. Environ. Chem., 2013 ; 95(8), 1416-1423.
- El-Kossi, A.E.A., Abdellah, M.M., Rashad, A.M., Hamed, S.A. The effectiveness of eveningprimroseoilevening primrose oil and alpha lipoic acid in recovery of nerve function in diabetic rats. J. Clin. Exp. Invest., 2011 ; 2(3):245-253.
- Reitman, S., Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol., 1957 ; 28(1), 56-63.
- McComb, R.B., Bowers, G.N. Study of optimum buffer conditions for measuring alkaline phosphatase activity in human serum. Clin. Chem., 1972 ; 18(2), 97-104.
- Szasz, G. A kinetic photometric method for serum γ-glutamyl transpeptidase. Clin. Chem., 1969 ; 15(2), 124-136.
- Doumas, B.T., Perry, B.W., Sasse, E.A., Straumfjord, J.V. Standardization in bilirubin assays: evaluation of selected methods and stability of bilirubin solutions. Clin. Chem., 1973 ; 19(9), 984-993.
- Peters, T. Total protein: Direct Biuret method. Clin. Chem. 1968 ; 14, 1147-1159.
- Trinder, P. Determination of glucose in body fluids. Ann. Clin. Biochem., 1969 ; 6, 24.
- Richmond, W. Preparation and properties of a cholesterol oxidase from Nocardia sp. andAnd its application to the enzymatic assay of total cholesterol in serum. Clin. Chem., 1973 ; 19(12), 1350-1356.
- Fossati, P., Prencipe, L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin. Chem., 1982 ; 28(10), 2077-2080.
- Warnick, G.R., Benderson, J., Albers, J.J. Dextran-sulfate-Mg2+ precipitation procedure for quantitation of high-density lipoprotein cholesterol. Cooper, G.R. eds. Selected methods of clinical chemistry; Vol. 10:91-99 American Association for Clinical Chemistry Washington, DC, 1983.
- Friedewald, W.T., Levy, R.I., Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem., 1972 ; 18(6), 499-502.
- Malstrom, B. andreassonAndreasson, L., Reinhammer, B. The enzymes. Enzymes. Academic Press, New York,1975 ; 507-579.
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med., 1963 ; 61, 882-888.
- El-Sheikh, E.A., Galal, A.A.A. Toxice ffects of sub-chronic exposure of male albino rats to emamectin benzoate and possible ameliorative role of Foeniculum vulgare essential oil. Environ. Toxicol. Pharmacol., 2015 ; 39(3):1177–1188
- Heikal, T.M., Mossa, A.H., Abdel Rasoul, M.A., Marei, G.I.Kh. The ameliorating effects of green tea extract against cyromazine and chlorpyrifos induced liver toxicity in male rats. Asian J. Pharmac. Clin. Res., 2013 ; 6(1):47-55.
- Thapa, B. R., Walia, A. Liver function tests and their interpretation. The Ind. J. Pediat., 2007 ; 74(7), 663-671.
- Tousson, E., Zaki, Z.T., Abu-Shaeir, W.A., Hassan, H. Methotrexate-induced hepatic and renal toxicity: role of L-carnitine in treatment. Biomed Biotechnol, 2014 ; 2(4), 85-92.
- Dobbs, N.A., Twelves, C.J., Gregory, W., Cruickshanka, C., Richards, M.A., Rubens, R.D. Epirubicin in patients withliverdysfunctionwith liver dysfunction: development and evaluation of a novel dose modification scheme. Eur. J. Cancer, 2003 ; 39(5), 580-586.
- Kew, M.C. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet, 2000 ; 355(9204), 591-592.
- Al-Attar, A. M. Attenuating effect of Ginkgo biloba leaves extract on liver fibrosis induced by thioacetamide in mice. BioMedRes. Int., 2012.
- Adeoti, O.T., Belonwu, D.C., Wegwu, M.O., Osuoha, J.O. Implication of Acute, Sub-chronic and ChronicExposure to Different Pesticides via Inhalation on Male Wistar Rats. Bioengineer. Biosci., 2017 ; 5(4), 74-85.
- Zidan, N.E.H.A. Hepato-and nephrotoxicity in male albino rats exposed to malathion and spinosad in stored wheat grains. Acta Biol. Hung., 2015 ; 66(2), 133-148.
- Mansour, M. K., El-Kashoury, A. A., Rashed, M. A., Koretem, K. M. Oxidative and biochemical alterations induced by profenofos insecticide in rats. Nature and Science, 2009 ; 7(2), 1-14.
- Adeniran, O.Y., Fafunso, M.A., Adeyemi, O., Lawal, A.O., Ologundudu, A., Omonkhua, A.A. Biochemical effects of pesticides on serum and urinological system of rats. J. Appl. Sci., 2006 ; 6(3), 668-672.
- Milošević, M.D., Paunović, M.G., Matić, M.M., Ognjanović, B.I., Saičić, Z.S. The ameliorating effects of selenium and vitamin C against fenitrothion-induced blood toxicity in Wistar rats. Environ. Toxicol. Pharmacol., 2017 ; 56, 204-209.
- Jayusman, P.A., Budin, S.B., Taib, I.S., Ghazali, A.R. The effect of tocotrienol-rich fraction on oxidative liver damage induced by fenitrothion. Sains Malaysiana, 2017 ; 46(9): 1603–1609.
- Elhalwagy, M.E., Darwish, N.S., Zaher, E.M. Prophylactic effect of green teapolyphenols against liver and kidney injury induced by fenitrothion insecticide. Pestic. Biochem. Physiol., 2008 ; 91(2), 81-89.
- Afshar, S., Heidari, R., Farshid, A.A., Ilkhanipour, M. Effect of oral administration of fenitrothion on biochemical and hematological parameters in rats. Pak. J. Biol. Sci.: PJBS, 2008 ; 11(13), 1742-1745.
- Attia, A.M., Nasr, H.M. Evaluation of the protective effect of omega-3 fatty acids and selenium on paraquat intoxicated rats. Slovak J. Anim. Sci., 2009 ; 42(4), 180-187.
- Rai, D.K., Rai, Prashant, K., Rizvi, Syed, I., Watal, G., Sharma, B. Carbofuran-induced toxicity in rats: Protective role of vitamin C. Exper. Toxicol. Pathol., 2009 ; 61(6), 531-535.
- Zari, T. A., Al-Attar, A. M. Therapeutic effects of olive leaves extract on rats treated with a sublethal concentration of carbendazim. Eur. Rev. Med. Pharmacol. Sci., 2011 ; 15(4), 413-426.
- Bhushan, C., Bhardwaj, A., Misra, S.S. State of Pesticide Regulations in India. New Delhi, Centre for Science and Environment.2013: 1-72.
- AbdElmonem, H.A. Assessment the effect of pomegranate molasses against diazinon toxicity in male rats. J. Environ. Sci. Toxicol. Food Technol, 2014 ; 8, 135-141.
- El-Demerdash, F.M., Jebur, A.B., Nasr, H.M. Oxidative stress and biochemical perturbations induced by insecticides mixture in rat testes. J. Environ. Sci. Health (B Pestic. Food Cont. Agric. Wastes), 2013 ; 48(7), 593-599·
- Ogutcu, A., Suludere, Z., Kalender, Y. Dichlorvos-induced hepatotoxicity in rats and the protective effects of vitamins C and E. Environ. Toxicol. Pharmacol., 2008 ; 26(3): 355-361
- Agbor, G.A., Oben, J.E., Ngogang, J.Y. Haematinic activity of Hibiscus cannabinus. Afr. J. Biotechnol., 2005 ; 4, 833-837.
- Yousef, M.I., Awad, T.I., Mohamed, E.H. Deltamethrin-induced oxidative damage and biochemical alterations in rat and its attenuation by Vitamin E. Toxicol., 2006 ; 227, 240–247.
- Lasram, M.M., Annabi, A.B., El Elj, N., Selmi, S., Kamoun, A., El-Fazaa, S., Gharbi, N. Metabolic disorders of acute exposure to malathion in adult Wistar rats. J. Hazard. Mater., 2009 ; 163(2-3), 1052-5.
- Ruckmani, A., Nayar, P.G., Konda, V.G.R., Madhusudha, N., Madhavi, E., Chokkaling, M., Meti, V., Sundaraval, S. Effects of inhalational exposure of malathion on blood glucose and antioxidants level in Wistar albino rats. Res. J. Environ. Toxicol., 2011 ; 5(5), 309-315.
- Rahimi, R., Abdollahi, M. A review on the mechanisms involved in hyperglycemia induced by organophosphorus pesticides. Pest. Biochem. Physiol., 2007 ; 88(2), 115-121.
- Pournourmohammadi, S., Ostad, S.N., Azizi, E., Ghahremani, M.H., Farzami, B., Minaie, B., Larijani, B., Abdollahi, M. Induction of insulin resistance by malathion: Evidence for disrupted islets cells metabolism and mitochondrial dysfunction. Pest. Biochem. Physiol., 2007 ; 88(3), 346-352.
- Valko, M., Leibfritz, D., Moncol, J., Cronin, M.T., Mazur, M., Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. The Inter. J. Biochem. Cell Biol., 2007 ; 39(1), 44-84.
- Ahmed, R.S., Seth, V., Pasha, S.T., Banerjee, B.D. Influence of dietary ginger (Zingiber officinalesRosc) on oxidative stress induced by malathion in rats. Food Chem. Toxicol., 2000 ; 38(5), 443-450.
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J., 2015 ; 15(1), 71.
- Taib, I. S., Budin, S. B., Ghazali, A. R., Jayusman, P. A., Mohamed, J. Fenitrothion alters sperm characteristics in rats: ameliorating effects of palm oiltocotrienol-rich fraction. Exper. Anim., 2014 ; 63(4), 383-393.
- Akhgari, M., Abdollahi, M., Kebryaeezadeh, A., Hosseini, R., Sabzevari, O. Biochemical evidence for free radical induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Human Exper. Toxicol., 2003 ; 22(4), 205-211.
- Al-Attar, A.M., Elnaggar, M.H., Almalki, E.A. Physiological study on the influence of some plant oils in rats exposed to a sublethal concentration of diazinon. Saudi J. Biol. Sci., 2018 ; 25(4), 786-796.
- Espinoza-Navarro, O., Ponce-Larosa, C., Bustos-Obregón, E. Organophosphorous pesticides: Their effects on biosentinel species and humans. Control and application in Chile. Int. J. Morphol., 2017 ; 35(3), 1069-1074.
- Innis, S.M. Dietaryomega 3 fatty acids and the developing brain. Brain Res., 2008 ; 1237, 35-43.
- Stark, A.H., Crawford, M.A., Reifen, R. Update on alpha-linolenic acid. Nutr. Rev., 2008 ; 66(6), 326-332.
- Cleland, L. G., James, M. J., Proudman, S. M. Fish oil: what the prescriber needs to know. ArthritisRes. Ther., 2005 ; 8(1), 202.
- Moghadasian, M.H. (2008). Advances in dietary enrichment with n-3 fatty acids. Crit. Rev. Food Sci. Nutr., 2008 ; 48(5), 402-410.
- Maki, K.C., Dicklin, M.R. Omega-3 fatty acid supplementation and cardiovascular disease risk: glass half full or time to nail the coffin shut? Nutrients, 2018 ; 10(7), 864.
- Su, K.P., Huang, S.Y., Chiu, C.C., Shen, W.W. Omega-3 fatty acids in major depressive disorder: a preliminary double-blind, placebo-controlled trial. Eur. Neuropsychopharmacol., 2003 ; 13(4), 267-271.
- Naliwaiko, K., Araújo, R.L.F., Da Fonseca, R.V., Castilho, J.C., Andreatini, R., Bellissimo, M.I., Ferraz, A.C. Effects of fishoilfish oil on the central nervous system: a new potential antidepressant. Nutr. Neurosci., 2004 ; 7(2), 91-99.
- Nemets, B., Stahl, Z., Belmaker, R.H. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psych., 2003 ; 159(3), 477-479.
- Yehuda, S., Rabinovitz, S., Mostofsky, D.I. Mixture of essential fatty acids lowers test anxiety. Nutr. Neurosci., 2005 ; 8(4), 265-267.
- Green, P., Hermesh, H., Monselise, A., Marom, S., Presburger, G., Weizman, A. Red cell membrane omega-3 fatty acids are decreased in nondepressed patients with social anxiety disorder. Eur. Neuropsychopharmacol., 2006 ; 16(2), 107-113.
- Leung, A.Y., Foster, S. Encyclopedia of Common Natural Ingredients Used in Food, Drugs, and Cosmetics. 2nd ed. Hoboken, NJ: Wiley-Interscience, 2003.
- Parker, H.M., Johnson, N.A., Burdon, C.A., Cohn, J.S., O’Connor, H.T., George, J. Omega-3 supplementation and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J. Hepatol., 2012 ; 56(4), 944-951.
- Faeh, D., Minehira, K., Schwarz, J.M., Periasamy, R., Park, S., Tappy, L. Effect of fructose overfeeding and fishoilfish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes, 2005 ; 54(7), 1907-1913.
- Lu, W., Li, S., Li, J., Wang, J., Zhang, R., Zhou, Y., Yin, Q., Zheng, Y., Wang, F., Xia, Y., Chen, K., Liu, T., Lu, J., Zhou, Y., Guo, C. Effects of Omega-3 Fatty Acid in Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Gastroenterol. Res. Pract., 2016 ; 1459790.
- Adeyemi, W.J, Olayaki, L.A. Diclofenac–induced hepatotoxicity: Low dose of omega-3 fatty acids have more protective effects. Toxicol. Rep., 2018 ; 5, 90-95.
- Moghadamnia, D., Mokhtari, M, Khatamsaz, S. Protective Effects of Omega Supplement on Induced Hepatic Mal-Function by Thioacetamide in Male Rats. Zahedan J. Res. Med. Sci., 2017 ; 19(3):e5796.
- Patten, A.R., Brocardo, P.S, Christie, B.R. Omega-3 supplementation can restore glutathione levels and prevent oxidative damage caused by prenatal ethanol exposure. The J. Nutr. Biochem., 2013 ; 24(5), 760-769.
- Wang, X., Zhao, X., Mao, Z.Y., Wang, X.M, Liu, Z.L. Neuroprotective effect of docosahexaenoic acid on glutamate-induced cytotoxicity in rat hippocampal cultures. Neuroreport, 2003 ; 14(18), 2457-2461.
- Zararsiz, I., Kus, I., Akpolat, N., Songur, A., Ogeturk, M, Sarsilmaz, M. Protective effects of ω‐3 essential fatty acids against formaldehyde‐induced neuronal damage in prefrontal cortex of rats. Cell Biochem. Func.: Cell. Biochem. Modul. Active Agents Dis., 2006 ; 24(3), 237-244.
- Martorell, M., Capó, X., Bibiloni, M.M., Sureda, A., Mestre-Alfaro, A., Batle, J.M., Llompart, I., Tur, J.A. Pons, A. Docosahexaenoic acid supplementation promotes serythrocyte antioxidant defense and reduces protein nitrosative damage in male athletes. Lipids, 2015 ; 50(2), 131-148.
