The Anti-Diabetic Effect of Hibiscus Cannabinus Extract on the Submandibular Salivary Gland of Alloxan-Induced Diabetic Albino Rats
Wael Elias
Associate Professor and Consultant in Oral and Maxillofacial Pathology, Chairman of the Oral Diagnostic Science Department, Faculty of Dentistry, King Abdul-Aziz University, Jeddah, Saudi Arabia.
Email: dreliasw @ gmail.com
ABSTRACT
Objectives: This study aims to investigate the anti-diabetic effect of Hibiscus cannabinus Linn leaf extract on the histological structure of the submandibular salivary gland in alloxan-induced diabetic albino rats. Materials and Methods: Three groups consisting of 10adult male albino rats each were used in this experiment. Group, I served as the control, whereas group II consisted of alloxan-induced diabetic rats. Finally, in group III, each alloxan-induced diabetic rat received an oral daily dose of methanolic extract of Hibiscus cannabinus Linn leaves equal to 400mg/kg body weight for four weeks after diabetes induction, using the same dose and like that of group II. In all groups, the rats were sacrificed four weeks after the experiment was initiated. The submandibular salivary glands were dissected, prepared, and stained with H&E for light microscopy examination. Results: Histopathological examination of the submandibular salivary glands in group II (the diabetic group) revealed a loss of the standard glandular architecture involving the entire structure of the glands, including the acini, duct system, connective tissue stroma, and blood vessels. Also, severe degenerative changes with an accumulation of numerous intracytoplasmic vacuoles affecting the serous acini and duct system were observed as compared to group 1. Most of these alterations and degenerative changes disappeared or were markedly decreased in group III (the diabetic group treated with Hibiscus cannabinus Linn extract). Conclusions: Hibiscus cannabinus Linn leaf extract had a noticeable anti¬diabetic effect on alloxan-induced diabetic alterations in the submandibular salivary glands of rats. Hence, Hibiscus cannabinus Linn leaves may be beneficial as a dietary supplement for reducing diabetes complications. Also, Hibiscus cannabinus Linn leaf extract can serve as a promising herbal medicine due to its effectiveness and safety.
Key words: Anti-Diabetic, Hibiscus Cannabinus, Alloxan-Induced, Salivary Gland.
INTRODUCTION
Many dangerous metabolic diseases affect the health and lives of human beings. One of the most serious metabolic diseases, spread widely throughout the world, is diabetes mellitus. It is one of the greatest public health challenges. [1-3]. Its hallmark is chronic hyperglycemia, which is due to a relative or total lack of insulin secretion from the pancreas, or from insulin resistance due to the body’s incapacity to respond appropriately to insulin, or both. Diabetes mellitus can affect individuals of all ages, though it is more common in adults. Recently, the World Health Organization (WHO) declared it to be a pandemic disease [4].
The long-term hyperglycemia that affects diabetic patients might lead to many adverse side effects, including progressive development of retinopathy with potential blindness as well as nephropathy with subsequent renal failure and neuropathy. Also, diabetic patients have a higher risk to develop cardiovascular and cerebrovascular diseases, myopathies, poor healing of wounds, and vascular disorders as well as poor oral and dental health [5–7].
Oxidative stress seems to play a crucial part in the pathophysiology of metabolic disorders such as diabetes mellitus and insulin resistance in humans. An association has been reported between several metabolic diseases and a general increase in oxidative damage and a reduction in oxidative defense [8].
Oxidative stress can be defined as the disturbance of the pro-oxidant antioxidant balance. It has been linked to the pathogenesis of diseases such as atherosclerosis, hypertension, and diabetes mellitus [9].
Because of the potentially detrimental effects of oxidative stress, even under normal physiological conditions, aerobic organisms have developed a complicated antioxidant system [10–12]. As of yet, there is no cure. To remain healthy, people with diabetes must manage their disease.
Like all medication, antidiabetic agents may have adverse effects. Hence, it is essential to replace these drugs with plant-based alternatives Recently, researchers throughout the world have focused on herbal medicines in an attempt to minimize such side effects [13–17].
It has been reported that many herbal medicines possess anti-diabetic properties. One of these herbal plants is Hibiscus cannabinus Linn. Hibiscus cannabinus is a warm-season annual herb fiber crop. It is native to Africa and has been commercially cultivated in many Asian countries [18, 19]. Hibiscus cannabinus Linn (family Malvaceae) is a known therapeutic anti-diabetic herb that is usually used as a dietary supplement [17, 20].
Phytochemical analysis has shown that the leaves of Hibiscus cannabinus extracts are rich in active phytoconstituents. Pharmacological experiments showed that these substances are known to exhibit a range of biological activities such as cytotoxic, anthelmintic, anti-inflammatory, antidiabetic, hypolipidemic, antioxidant, immunological, hematinic, and hepatoprotective effects due to their ability to scavenge free radicals [17]. Thus, this study aimed to determine the anti-diabetic effect of Hibiscus cannabinus Linn leaf methanolic extract on the histological structure of the submandibular salivary glands of alloxan-induced diabetic albino rats.
MATERIALS AND METHODS
This study was conducted on 30 healthy adult male albino rats that were three months of age and that had an average body weight of between 200-250 g. The animals were placed in individual cages and were fed a balanced diet. Water was given ad-libitum. The animals were divided into three groups (10 animals each) as follows:
Group I or the control group consisted of 10 healthy untreated rats that received water.
Group II or the diabetic group consisted of 10 rats, each of which received a single intraperitoneal injection of 150mg/kg bodyweight of alloxan monohydrate to induce diabetes.
Group III or the treated group consisted of 10 alloxan-induced diabetic rats using the same dose as that of group II. Additionally, each animal received an oral daily dose of methanolic Hibiscus cannabinus Linn leaf extract (400 mg/kg body weight) using an oro-pharyngeal metallic curved tube for four weeks after induction of diabetes. After completion of the experiment, the rats in all three groups were sacrificed by intracardiac injection of a high dose of an anesthetic agent (phenobarbital). The submandibular salivary glands of the rats in all three groups were carefully dissected and prepared for histopathology.
Tissue processing for light microscopy: The dissected glands were fixed in 10% calciferol, dehydrated in increasing concentrations of alcohol, cleared in xylol, and embedded in paraffin. Sections, which were six microns thick, were deparaffinized and the slices were hematoxylin and eosin stained for examination under a light microscope.
RESULTS
Histopathological Results
Group I (Control group):
A thin fibrous C.T. capsule surrounded the submandibular salivary glands of these rats. The serous acini appeared rounded and small in size, with narrow lumens. These acini had a single layer of pyramidal-shaped cells with granular basophilic cytoplasm and rounded prominent, deeply stained and basally situated nuclei. The granular convoluted tubules (GCTs) were the most prominent structure in the salivary glands and were lined with tall columnar cells with rounded or oval basally situated nuclei and abundant eosinophilic granules. The striated ducts had a single layer of columnar cells with rounded, centrally located, and darkly stained nuclei and eosinophilic cytoplasm with apparent acidophilic basal striations. The excretory ducts were seen between the lobes of the gland surrounded by thin fibrous connective tissue stroma. These ducts were lined with a pseudostratified columnar epithelium with rounded or oval deeply stained nuclei appearing at different levels. Healthy blood vessels with numerous RBCs were also seen (Fig. l).
Group II (Diabetic group):
The diabetic rat submandibular salivary gland showed severe pathological changes and seemed to have lost its normal glandular architecture. The serous acini appeared irregular in shape and smaller in size, with variable grades of degenerative changes. Some areas showed a loss of acinar architecture (amalgamation) with hyperchromatic nuclei, while other acini presented numerous intracytoplasmic vacuolizations. The GCTs revealed areas of degeneration in their epithelial lining, with a marked reduction in the apical content of the eosinophilic granules and the presence of intracytoplasmic vacuolization. The striated ducts decreased in size, and their cells showed areas of degeneration and perinuclear spaces with indistinct basal striations. The excretory ducts revealed areas of degeneration and perinuclear spaces in their cell lining, with a dilated lumen and retained secretion. The blood vessels appeared to be dilated, congested, and engorged with RBCs within the thickened fibrous C.T. stroma. Chronic inflammatory cell infiltrates could also be seen (Fig. 2).
Group III (Treated group):
The structure of the submandibular salivary glands of group III (diabetic rats that received Hibiscus cannabinus Linn leaf methanolic extract) was markedly improved than that of the diabetic group. However, the submandibular salivary glands of group III rats showed limited histological alterations than that of group I or control rats. The acini had relatively restored their standard architecture, though they still had hyperchromatic nuclei. The cytoplasmic basophilia of the acinar cells had considerably increased, though still fewer intracytoplasmic vacuolizations were detected in some acinar cells. In terms of the duct system, the GCTs showed usual lining with a moderate increase in the apical eosinophilic granular content of their cells compared to those in the diabetic group. The striated ducts appeared similar to those of group I rats, with a normal lining of columnar cells with rounded centrally placed and darkly stained nuclei and eosinophilic cytoplasm with distinct basal striations. The excretory ducts appeared to have the normal lining of a pseudostratified columnar epithelium with a dilated lumen and retained secretion. Dilated blood vessels that were congested and engorged with RBCs were also seen (Fig.3).
FIG. (1) A photomicrograph of rat submandibular salivary gland of a group I (control) rat showing normal histology of the gland with serous acini (a), GCTs (g), striated ducts (s), excretory ducts (e), thin fibrous C.T. stroma (f), and blood vessels (b) (H&E, X.200).
FIG. (2) A photomicrograph of the submandibular salivary glands of a diabetic (group II) rat showing small-sized irregular serous acini with decreased cytoplasmic basophilia (a), intracytoplasmic vacuolizations (v), hyperchromatic nuclei (n), amalgamation of serous acini (m), decreased eosinophilic granules of the GCTs (g), degeneration and indistinguishable basal striations of the striated ducts (s), degeneration and perinuclear spaces in the excretory ducts with dilated lumen and retained secretion (e), blood vessels that are dilated, congested, and engorged with RBCs (b), thickened fibrous C.T. stroma (f), and chronic inflammatory cells (i) (H&E, X.200).
FIG. (3) A photomicrograph of rat submandibular salivary glands of the treated group (group III) showing a normal architecture of serous acini with normal basophilia (a) and a few intracytoplasmic vacuolizations (v), GCTs with moderate eosinophilic granules (g), and excretory duct with dilated lumen and retained secretion (e), fibrous C.T. stroma (f), and blood vessels that are dilated, congested, and engorged with RBCs (b) (H&E, X.200).
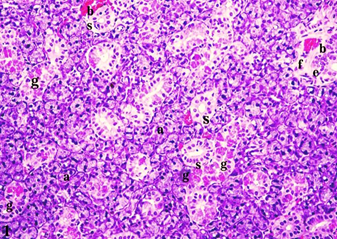
Fig. (1) A photomicrograph of rat submandibular salivary gland of the control group (group I) showing normal histology of the gland with serous acini (a), GCTs (g), striated ducts (s), excretory ducts (e), thin fibrous C.T. stroma (f), and blood vessels (b) (H&E, X.200).
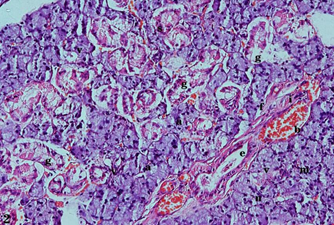
Fig. (2) A photomicrograph of rat submandibular salivary glands of the diabetic group (group II) showing small-sized irregular serous acini with decreased cytoplasmic basophilia (a), intracytoplasmicvacuolizations(v), hyperchromatic nuclei (n), amalgamation of serous acini (m), decreased eosinophilic granules of the GCTs (g), degeneration and indistinct basal striations of the striated ducts (s), degeneration and perinuclear spaces in the excretory ducts with a dilated lumen and retained secretion (e), blood vessels that are dilated, congested, and engorged with RBCs (b), thickened fibrous C.T. stroma (f), and chronic inflammatory cells(i) (H&E, X.200).
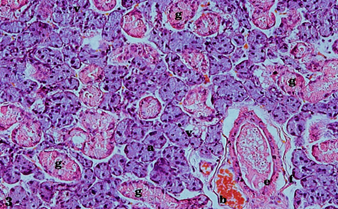
Fig. (3) A photomicrograph of rat submandibular salivary glands of the treated group (group III) showing a normal architecture of serous acini with normal basophilia (a) and a few intracytoplasmicvacuolizations (v), GCTs with moderate eosinophilic granules (g), and excretory duct with dilated lumen and retained secretion (e), fibrous C.T. stroma (f), and blood vessels that are dilated, congested, and engorged with RBCs (b) (H&E, X.200).
DISCUSSION
Diabetes mellitus is a metabolic disease that is posing one of the greatest threats to human health and life. Because of the massive experience of past generations, nowadays, all the world’s civilizations have extensive knowledge of herbal medicine. Recently, it was reported that most people have focused on using plants for therapy and disease prevention. Additionally, people have been using herbal therapy to ameliorate, or at least minimize, the side effects of synthetic drugs.
Plants are valuable sources of several secondary metabolites, which are used for diverse purposes, primarily as pharmaceuticals [19]. The phytochemical analysis of Hibiscus cannabinus revealed the presence of active phytoconstituents such as flavonoids, polyphenols, tannins, steroids, alkaloids, and saponins. Pharmacological studies of these contents demonstrated that Hibiscus cannabinus possesses antidiabetic, hypolipidemic, antioxidant, hematinic, and hepatoprotective effects [20].
This study was designed based on the assumption that the methanolic extract of Hibiscus cannabinus Linn leaves has an anti-diabetic and antioxidant effect on the submandibular salivary glands of alloxan-induced diabetic albino rats. This assumption was based on several points of view, including that hibiscus anthocyanins were extracted from the dried leaves [17, 20], manifest vigorous antioxidant activity in a liposomal system, and could improve hyperglycemia and insulin sensitivity in diabetic rodents [10–12]. The health benefits of fruits and vegetables may be explained in part by the presence of polyphenols, which maintain several biological activities, including antioxidant and free radical-scavenging properties, an anti-aggregatory platelet property, and the inhibition of vascular smooth muscle cell proliferation [21].
This study used a dose of methanolic extract of Hibiscus cannabinus Linn leaves equal to 400 mg/kg body weight. This is per the toxicity study which explained that the methanolic extract of Hibiscus cannabinus was nontoxic up to 5g/kg body weight [17].
Our analyses showed that the submandibular salivary glands of group I (control) rats had a normal histological structure. Meanwhile, in group II (the diabetic group), most of the serous acini revealed extensive intracytoplasmic vacuolizations. This is consistent with the large vacuoles that Cutler et al. [22] observed in the cytoplasm of many of the acinar cells in diabetic rat submandibular glands. Also, they appeared similar to those demonstrated by Anderson and Johnson [23] and Takai et al. [24] in diabetic parotid and submandibular glands, respectively. The authors explained that the presence of these vacuoles could be attributed to the intracytoplasmic lipid accumulation within the cells. However, a large proportion of the lipid seems to dissolve and be removed during tissue fixation and processing. Also, the acinar cells showed decreased cytoplasmic basophilia. A possible explanation can be deduced from the findings of Takai et al. [24], who demonstrated degenerative changes, including a decrease in the quantity of rough endoplasmic reticulum and the disappearance of the cristae of mitochondria in the acinar cells of the submandibular glands of diabetic rats. The observed nuclear hyperchromatism in the diabetic acinar cells in this study is similar to that reported by Gorlin and Goldman [25] and Reuterving et al. [26]. They attributed this change to increased gland function caused by increased food intake and mastication in diabetic rats, or to the repair processes in the gland.
In this study, the GCTs, striated ducts, and excretory ducts showed degeneration in the cellular lining as well as occasional vacuoles. These findings agree with the results of Takai et al. [24], Anderson and Garrett [27], and Reuterving et al.[26], who demonstrated the presence of vacuoles and lipid droplets in the intercalated ducts of the diabetic submandibular gland. On the other hand, Cutler et al.[22] reported that the intercalated and striated ducts of the submandibular glands of STZ-diabetic rats did not change throughout the experimental period. Also, Anderson and Johnson [23] found no intracellular vacuolizations in the duct cells of diabetic parotid glands. In the present work, the GCTs exhibited a marked reduction in apical eosinophilic granular content. This finding coincides with the results of Kasayama and Oka [28] and Anderson et al.[29], who investigated diabetic submandibular glands. This result could be attributed to impairment in the function of the Golgi complex according to the findings of Kasayama and Oka [28] and Anderson et al. [29].
In our study, dilatation in the lumen was noticed in the excretory ducts of the diabetic glands, which is similar to the results reported by Gorlin and Goldman [25]. Similarly, Reuterving et al. [26] reported duct dilatation in the salivary glands of recently diagnosed diabetic rats. In the present investigation, the chronic inflammatory cell infiltrate seen in the C.T. septa coincides with the findings of Cutler et al. [22], who demonstrated the presence of occasional inflammatory cells in the C.T. It is plausible that inflammation occurs due to the release of products upon the death of the secretory cells. Also, this agrees with a large number of chronic inflammatory cells that were noticed to be infiltrating the C.T. stroma around the acini and ducts in the minor salivary glands in diabetic patients. The increase in the fibrous C.T. stroma observed in the present work agrees with the findings of Schnider and Kohn [30, 31], who noted the presence of several vascular changes and an apparent acceleration of the aging process in the presence of diabetes mellitus.
Histopathological examination of the rat submandibular salivary gland of treated rats (group III) revealed that the entire glandular structure was markedly improved compared to that of the diabetic group, though the glandular architecture was not entirely similar for that of the control group. The acini revealed normal basophilia, though some still had a few intracytoplasmic vacuolizations and hyperchromatic nuclei. The GCTs restored their structure with moderate content of their characteristic eosinophilic granules. The striated ducts restored their normal lining with the characteristic acidophilic basal striations. The excretory ducts had their normal lining and dilated lumen with retained secretion. Dilated blood vessels engorged with RBCs were noted. Also, there was thin fibrous C.T. stroma than that observed in the diabetic group. These results are consistent with those of several studies confirming the anti-diabetic, antioxidant effects of Hibiscus cannabinus Linn [17, 20]. This finding of the extract’s antidiabetic effect supports the results of Rajan et al. [17] (2011), who reported that the oral administration of 400 mg/kg alcoholic extract of Hibiscus cannabinus leaves for 15 days significantly lowered blood glucose in hyperglycemic rats [17]. Also, the antioxidant effect of Hibiscus cannabinus extract could be attributed to its free-radical scavenging properties and its ability to protect DNA from oxidative damage and the inhibiting gelatinolytic activity of collagenase types I and II. Also, the methanolic extract of the leaves of Hibiscus cannabinus Linn, as an antioxidant, could decrease the expression of NFxB in the submandibular salivary glands of diabetic rats, based on the fact that polyphenols indirectly function as antioxidants down-regulating “pro-oxidant” enzymes (such as iNOS, lipoxygenases, and xanthine oxidase) and inhibiting NF-xB (Frei & Higdon, 2003). Hence, the extract has strong antioxidant activity in a liposomal system and could ameliorate hyperglycemia and insulin sensitivity in diabetic mice [10–12]. This might explain the extract’s efficiency in preventing or minimizing the incidence of diabetes-induced degenerative changes in this group. This explanation aligns with previous studies that investigated the mechanism by which oxidative stress can result in diabetes mellitus [8, 9]. Cumulatively, Hibiscus cannabinus extracts can be used as potential functional foods to minimize oxidative stress, free radical-induced DNA damage, and bone disorders like osteoarthritis [32].
Also, the hypolipidemic effect of 50% hydroalcoholic extract of Hibiscus cannabinus leaves was evaluated in the model involving rats fed a high-fat diet. The extract exhibited strong dose-dependent antihyperlipidemic activity. The administration of 400mg/kg of the extract caused a significant reduction in the levels of serum TC, TG, LDL-C, VLDL-C, and TBARS. The extract also considerably prevented hepatic fat accumulation in hyperlipidemic rats [33]. This could explain the observed reduction in intracytoplasmic vacuolization and lipid accumulation in the acini and ducts of the submandibular gland in this group of the present study.
Moreover, the observed reduction in the inflammatory cells in this study could be related to the immunological activity of the methanolic extract of Hibiscus cannabinus leaves. The latter significantly suppressed the secretion of TNF-alpha production and the mRNA expression of interleukin (IL)-3 and IL-12 in the RAW264.7 cells, stimulated by lipopolysaccharide (LPS, 2.5 microg/ml) and diminished the inflammatory mediators (i.e., nitric oxide, reactive oxygen species, and prostaglandin E2) [34].
Furthermore, the dilated blood vessels that were congested and engorged with RBCs in the present study could be explained on the basis that the leaf extract of Hibiscus cannabinus possesses a hematinic effect and could induce a significantly (P<0.050 higher red blood cell count, hemoglobin level, and packed cell volume (which had originally been reduced by phenylhydrazine administration) within one week of therapy. The presence of macrocytosis turned toward normal as the animals recovered from the anemic condition [35].
CONCLUSIONS
This study proved that Hibiscus cannabinus Linn had a potentially antidiabetic effect against alloxan-induced diabetic changes in the rat submandibular salivary gland. Hence, Hibiscus cannabinus Linn leaves can be used as a dietary supplement in diabetic patients. Also, they can be used as a promising herbal medicine due to their effectiveness and safety.
REFERENCES