Biologically Active Complex for Metabolism Normalization in People with Angioneurosis: Properties and Efficacy
Podzorova Galina Anatolievna, Boisjoni Tokhiriyon*, Donskova Lyudmila Aleksandrovna, Poznyakovsky Valeriy Mikhaylovich
Ural State University of Economics, Institute of Commerce, Food Technology and Service, Russia.
ABSTRACT
The paper deals with the scientifically based composition of a new food supplement. The specialized product improves metabolism in people with nervous system disorders, its ingredients having a synergetic effect. One tablet of the food supplement contains, mg: Gotu Kola (fruit) – 50 , L-glutamine acid - 50, motherwort - 25, lecithin - 25, gamma-aminobutyric acid - 25, calcium carbonate - 25, magnesium oxide - 25, choline bitartrate - 20, guarana - 16.5, ginkgo biloba (extract) - 15, hawthorn (fruit) - 15, ginseng (root) - 13, inositol - 8, L-methionine - 8, L-tyrosine - 7.5, L-phenylalanine - 7.5, L-caratin - 5, vitamin B3 - 5, B5-2.5, DNase - 4, RNase - 4, vitamin B6 - 1, vitamin B1 - 0.5, folic acid - 0.2, vitamin B12 - 00005. The authors provide biochemical characteristics of the supplement active substances to establish its functional properties and regulated quality indicators, including nutritional value, as well as a possible mechanism of metabolism normalization. The safety criteria comply with regulatory documents requirements, which have been proved by sanitary-hygienic and sanitary-toxicological studies.
The functional properties of the specialized product and its efficacy are confirmed by conducting field trials with a representative group of patients with angioneurosis. Taking two tablets (recommended daily dose) provides the body with the following nutrients intake (in parentheses - percentage of the recommended daily intake): vitamin B1 – 1mg (67%); Vitamin B3 - 10 mg (50%); Vitamin B5 - 5 mg (100%); Vitamin B6 - 2.0 mg (100%); Vitamin B9 - 0.4 mg (200%); vitamin B12 - 0.001 mg (30%); magnesium - 30 mg (8%), flavone glycosides (quercetin, campherol, isorhamnetin) - 2 mg (6%). The product composition and manufacturing technology have been tested and implemented at the enterprises of the company ArtLife (Tomsk), which are certified following the requirements of the international standards of the ISO 9001, 22000 series, and GMP rules. That ensures product quality and functional properties stability.
Key words: Biologically active food supplement, composition, manufacturing technology, nutritional value, functional properties, efficacy.
INTRODUCTION
Specialized products, including biologically active food supplements, are affordable and, at the same time, effective in improving people’s nutrition and health. They are especially relevant in the prevention and combination therapy of common diseases, special attention being paid to the nervous system diseases [1-12]. Quality of life includes the mental, physical, emotional and social feeling of well-being, and reflects patients’ mental evaluation of their status of health and their response to it [13]. Health is a human right [14, 15]. In each health system, patients naturally expect to receive the required services at the right quality and time [16].
The nervous system perceives and analyzes information about the surrounding world and ensures the coordinated functioning of a complex biochemical ensemble of the body mechanisms [17, 18]. This is especially true of higher nervous activity and its center - the brain, which implements their functions through emotions, speech, memory, intellectual development, and other manifestations of metabolic transformations.
Throughout life, nerve cells need nutritional support, provided by several essential nutrients (phospholipids, amino acids, vitamins, etc.), as well as by impeccable vascular bed functioning, which ensures the flow of biologically active substances to the nervous tissue. Food supplements can provide this multifactorial support to the nervous system, which is essential in our age of techno genic overload.
Research objectives were to develop a new specialized product composition and manufacturing technology, determine its regulated quality indicators, and prove its functional properties and efficacy by clinical trials.
MATERIALS AND METHODS
The materials used are incoming raw materials, semi-finished products, laboratory and experimental samples of dietary supplements, representative groups of patients with angioneurosis. Common and specific methods of testing quality, safety, and functional properties were used [19, 20].
A new type of specialized product has been developed, a dietary supplement. The product composition is scientifically based, the components having a synergetic effect on the metabolic processes of nervous tissue. The product composition is as follows, mg per tablet: Vitamin B1 - 0.1; Vitamin B12 - 0.0001; Folic acid - 0.1; Vitamin B6 - 0.2; L-carnitine - 1; Vitamin B3 - 1; Vitamin B5 - 1; L - tyrosine - 1.5; L-Phenylalanine - 1.5; DNase - 0.8; RNase - 0.8; Inositol - 1.6; L-methionine - 1.6; Korean ginseng (root) - 2.6; Ginkgo biloba (extract) - 3; Hawthorn (fruit) - 3; Guarana - 3.3; Choline bitartrate - 4; Motherwort (grass) - 5; Lecithin - 5; Gamma-aminobutyric acid - 5; Calcium Carbonate - 5; Magnesium oxide - 5; Gotu kola (fruit) - 10; L-Glutamic acid - 10.
Biochemical characteristics of individual biologically active substances of the dietary supplement composition are given to determine its functional properties and a possible mechanism for metabolism normalization.
The plant extracts and bioactive substances of the biological complex maintain the natural balance of nutrients required by the nervous tissue. Gotu Kola, ginseng, and guarana active substances have a tonic effect on the body, glutamic acid and trace elements (calcium and magnesium) are involved in the regulation and formation of nerve impulses, and energy supply of nerve cells. Gamma-aminobutyric acid and motherwort protect nervous tissue from super excitation that interferes with intense brain functioning. The product plant ingredients (ginkgo biloba extract, horse chestnut, and hawthorn fruit) improve microcirculation, prevent congestion in the brain blood pool, and high blood pressure. A combination of nutrients, including inositol, B vitamins, enzymes, and amino acids, stabilizes energy metabolism and regulates the molecular processes that underlie memory and attention.
RESULTS AND DISCUSSION
The developed product belongs to para pharmaceuticals. Their functional properties are demonstrated by the following diagram (Figure 1).
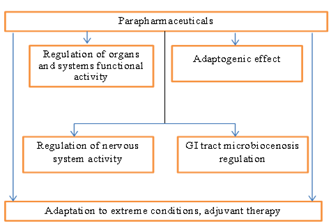
Figure 1. Functional properties of food supplements- parapharmaceuticals
According to the Quality and Technology Guidelines, the manufacturing technology consists of the following main stages: incoming control of raw materials followed by putting a green band on the identification label; dosing ingredients; sifting raw materials through a sieve No. 4; blending to a homogeneous mass for 60 minutes; wet granulation with a press machine through a die with a hole diameter of 1 mm. An aqueous lecithin solution is used as a humidifier, its amount is 55% of the dry weight. Wet granulate should be uniform and of the same color. The temperature of wet granules when exiting the die should be 40 ° C maximum. The next stage of the process is granulate drying, which is carried out in ovens at the temperature of 60 ° C maximum. The optimum drying time is 1.5–2 hours. The residual moisture of dried granules should be 8–9%. Dry granules are weighed. The stage of dry granulation is carried out with a Fitz Mill granulator No. 1, 2. Then tableting and tablet dedusting stages follow. The tablet mass is pressed with a rotary tablet machine PTM-E 150 (KILLIAN). The working pressure during pressing is 50 n maximum. Tablet average mass and tensile strength are checked every 30 minutes. Tablets cannot have chips, cracks, stains, foreign inclusions, stratification and must have a smooth surface. After dedusting tablets are put into containers and go to the film coating stage. Water-soluble film coating is applied with ACCELOCOTA-350 unit. The coating mass is 3.5 % of tablet weight. This stage is followed by tablet quality inspection and sorting; finally packing and packaging. Finished products are tested for compliance with established requirements. At each stage of the process, labeling is carried out. Labels show the product name, its quantity, batch number, and manufacturing date. All this information is recorded in a tracking sheet.
The product underwent organoleptic, physico-chemical, and microbiological testing during its manufacturing process and storage, which allowed establishing its regulated quality indicators. The packaged product was kept in a dry place at room temperature for 39 months. We provide results of sanitary - hygienic (table 1) and sanitary-toxicological (table 2) testing after the storage expiration date.
Table 1. Food supplement- sanitary-hygienic safety indicators
|
Microbiological indicators |
Indicators |
Permissible level by reference document |
Test results |
|
MAFAM (CFU/g, maximum) |
10000 |
1000 |
|
|
CGB (coliforms) in 0.1g |
Prohibited |
None |
|
|
E.coli in 0.1 g. |
Prohibited |
None |
|
|
Staphylococcus aureus in 1.0 g |
Prohibited |
None |
|
|
B. cereus (CFU/g, maximum) |
200 |
Maximum 10 |
|
|
Pathogenic microorganisms, including salmonella |
In 10.0 g. Prohibited |
None |
|
|
Yeasts, molds (CFU/g, maximum) |
100 |
Maximum 10 |
Table 2. Food supplement - sanitary - toxicological indicators
|
Indicator group |
Tested indicator |
Permissible level by reference document |
Test results |
|
Radionuclides |
Cesium-137 (bq/kg, maximum |
200 |
0.85 |
|
Strontium -90 (bq/kg, maximum) |
100 |
16.00 |
|
|
Toxic metals |
Lead (mcg/kg, maximum) |
6.0 |
0.44 |
|
Arsenic (mcg/kg, maximum) |
0.5 |
0.012 |
|
|
Cadmium (mcg/kg, maximum) |
1.0 |
Maximum 0.002 |
|
|
Mercury (mcg/kg, maximum) |
0.1 |
0.017 |
|
|
Pesticides |
HCCH (total isomers) (mcg/kg, maximum) |
0.1 |
Maximum 0.005 |
|
DDT and its metabolites (mcg/kg, maximum) |
0.1 |
Maximum 0.005 |
|
|
Heptachlor (mcg/kg, maximum) |
Prohibited (maximum 0.002) |
Maximum 0.002 |
|
|
Aldrin (mcg/kg, maximum) |
Prohibited (maximum 0.002) |
Maximum 0.002 |
The data provided in the tables prove the product safety and allow establishing its regulated quality indicators, including nutritional value (tables 3, 4).
Table 3. Food supplement - organoleptic and physico-chemical indicators
|
Indicator |
Description |
|
Appearance |
Coated oval tablets |
|
Shell color |
Blue |
|
Under shell color |
Shades of gray-green, blotches are acceptable |
|
Taste and odor |
Specific |
|
Tablet average mass, g |
0.47-0.53 |
|
Tensile strength, H, minimum |
90 |
|
Friability strength, %, minimum |
97 |
Table 4. Food supplement - nutritional value
|
Indicator, mg/tablet |
Content |
|
В1 |
0.5 (0.4-0.6) |
|
В3 |
5 (4.5-5.5) |
|
В6 |
1.0 (0.8-1.2) |
|
В5 |
2.5 (2.1-3.0) |
|
В9 |
0.2 (0.17-0.24) |
|
Gingosides |
0.3 (0.2-0.5) |
|
Flavonoids |
2.5 (2.0-3.0) |
|
Carnitine |
5 (4.2-6.0) |
|
Glutamic acid |
50 (42.5-60) |
|
Magnesium |
15 (13.5-16.5) |
The regulated indicators of nutritional value determine the product's functional properties. The product efficacy has been proved by including it into the combination therapy of patients with angioneurosis.
100 volunteers aged 38 - 50 were under observation. They were divided into two groups of 50 people: the first group (main group) took traditional vasoactive drugs and the food supplement; the second group (control group) took only pharmaceutical drugs.
The administration of the food supplement caused more significant headache abatement than the control group patients recorded. Thus, at the end of the treatment period, the number of the main group patients, complaining of headaches, decreased by 50%, while in the control group this number only decreased by 25 %. In the main group, the frequency of complaints about reduced mental function and well-being decreased by 2 times. 80% of the main group patients showed more initiative and 84% showed better attention. In the control group, the corresponding numbers were 50% and 60% (Figure 2).
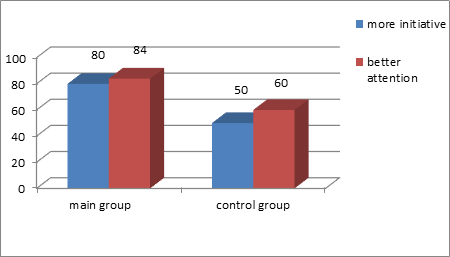
Figure 2. Positive dynamics of nervous system functioning in patients with angioneurosis
Patients, who took demonstrated normalization of the higher nervous activity and cerebral cortex functions, recording a significantly better mood and muscle tone. Their emotional status and muscle tone improvement was twice as good as that of the control group patients.
CONCLUSION
The obtained results prove that the food supplement is effective in treating angioneurosis:
The recommended product intake is as follows: 1 tablet twice a day with food in combination with Trental. The treatment period is 4 weeks. The maintenance dose is 1 tablet per day for 2-3 months.
Taking two tablets (recommended daily dose) provides the body with the following nutrients intake, in mg (in parentheses - percentage of the recommended daily intake): vitamin B1 - 1 (67); Vitamin B3 - 10 (50); Vitamin B5 - 5 (100); Vitamin B6 - 2.0 (100); Vitamin B9 - 0.4 (200); vitamin B12 - 0.001 (30); magnesium - 30 (8).
The tableting manufacturing technology provides high stability of the formulation ingredients active substances due to no aeration and an insignificant amount of residual moisture, which prevents oxidation.
The product shelf life is 3 years with three months’ allowance at room temperature in a dry place out of children’s reach.
The specialized product is produced at the enterprises of the company "Artlife" (Tomsk). An integrated quality and safety system was developed and implemented following the requirements of international standards of the ISO 9001, 22000 series, and GMP rules [19, 21-25]. The system efficacy was confirmed, which ensures the stability of the product quality, safety, and functional properties.
ACKNOWLEDGEMENT
The study was performed at the premises of the Department of Therapy of the Advanced and Post-graduate Training Faculty of the Siberian State Medical University under the direction of E.I. Beloborodova, Holder of the Habilitation degree in Medicine, Professor, Honored Doctor of the Russian Federation, to whom the authors would like to express their deep gratitude.
Conflict of interest
There was no conflict of interest among the authors.
REFERENCES