Effect of aqueous Salvia officinalis extract on Aluminum chloride-induced neurotoxicity in female rats
Amina Boussadia1*, Omar Kharoubi1, Zakia Lahouel1, Abderrezzak Benglia2, Abdelkader Aoues1
1 Laboratory of experimental Bio-Toxicology, Bio-Depollution, and Phytoremediation, Department of Biology, Faculty of natural and life sciences, University Oran1 ABB, Oran, Algeria.
2Laboratory of Biochemistry, Regional Military University Hospital of Oran, Algeria.
*Email: Aminaboussadia @ yahoo.fr
ABSTRACT
The neurotoxicity of Aluminum has been discovered in several experiments. It is a neurotoxic element involved in the etiology of some neurodegenerative diseases. Hence, the present study was designed to evaluate the neuroprotective effect of Salvia officinalis aqueous leaf extract (SAE) on the behavioral, histological, and biochemical alterations caused by Aluminum chloride in female Wistar rats. Chronic aluminum chloride (AlCl3) exposure developed behavioral deficits by decreasing locomotor activity and caused a significant reduction of spontaneous alternation. Besides, AlCl3 exposure showed a significant decrease in Acetylcholinesterase activity (-54.49%), Catalase activity (-42.48%), and a significant increase in the mean concentration of Malondialdehyde (MDA) compared to control group. Histological alterations were observed in the brain of aluminum chloride exposed group, which explains the neurodegenerative effect of aluminum on rat brain histology. SAE treatment improved behavioral and histological changes in AlCl3-induced rats, attenuated biochemical alteration by recording an increase in Acetylcholinesterase activity at 73.68% and enhanced catalase activity by 62.7%, as well as a decrease in the MDA level of -86.81% compared to the AlCl3 group. The current study demonstrates that Salvia officinalis can be administered as a food additive to protect against the neurotoxicity of AlCl3 and to ameliorate behavioral and oxidative status.
Key words: Aluminium, Salvia officinalis extract, Brain, Behavior test, Histological study.
INTRODUCTION
Aluminum is widely distributed in the ambient environment and used in various fields [1, 2]. It is present in several consumer products, including cooking utensils, drugs, cosmetics, and food additives [3-5]. However, it has no specific biological or physiological function [6] and can even induce neurotoxicity [7, 8].
Aluminum is a neurotoxic element involved in the etiology of some neurodegenerative disorders like Alzheimer's diseases [9, 10], Parkinson’s diseases [11] dialysis encephalopathy, amyotrophic lateral sclerosis, and other chronic neurodegenerative diseases [12, 13].
The exposure to aluminum in the long-term could affect the neurocognitive function and dopaminergic metabolism; it could cause dendritic atrophy and behavioral deterioration [14] and may be associated with the neurofibrillary tangles and amyloid plaques of patients with Alzheimer's disease [15]. It is well known that aluminum affects the hippocampus [16] and cortex regions [17] more severely than the other areas of the central nervous system (CNS).
The effects of aluminum on the CNS were largely studied; it can exert a harmful effect on the activity of choline acetyltransferase especially in the hippocampus [18]. Moreover, aluminum was shown as being able to not only cross the hematoencephalic barrier [19] but also increase its permeability [20].
Salvia officinalis L., (sage) belongs to family Lamiaceae, herbaceous plants cultivated worldwide. It’s well known aromatic and medicinal vegetal species largely employed in flavoring and seasoning food which grows in countries bordering the Mediterranean Sea [21, 22]. Its botanical name refers to its medicinal properties: Salvia is derived from the Latin word "salvage" and means "to cure".
Salvia officinalis L. is used as a traditional herbal medicine against various diseases, such as gastric disturbances and inflammatory processes [23]. It presents many pharmacological properties, most of them associated with its largess polyphenol content [24-26].
Various extracts of Salvia have been evaluated for biological effects, and their neuroprotective [27, 28], antimicrobial [29], anti-inflammatory [30, 31], immunomodulatory, antioxidant [32, 33], spasmolytic, anticancer [34, 35], and cholinergic [21, 36, 37] effects.
Earlier studies demonstrated the presence of carnosol, rosmarinic acid, oleanolic acid, ursolic acid, and in the leaves of S.officinalis [38]. Some properties of these compounds have already been described, such as their antioxidant activity [39, 40].
Rosmarinic and carnosic acids, which have antioxidant properties, are present in high concentrations in the sage extract [41]. The inhibition of lipid peroxidation by rosmarinic acid and carnosol [21] as well as the anti-inflammatory and pro-inflammatory activities of ursolic acid [42, 43] have already been described, too.
The present study aimed to determine the neurobiochimic and antioxidant properties of the aqueous extract of Salvia officinalis against chronic exposure to aluminum chloride and to investigate its potential against Aluminum-induced neurodegenerative changes in Wistar rats.
MATERIAL AND METHODS
Collection and identification of plant materials
The fresh leaves of Salvia officinalis were collected from the region of Mostaganem, North-West of Algeria during March; 2016. The taxonomic identity of the plant was confirmed by Professor HADJAJ in the Botany Laboratory of Oran1 University, Algeria. The plant materials were washed with distilled water than were dried for one month at room temperature.
Preparation of aqueous extract
Aqueous extract of sage was obtained by extraction of powder leaves (50 g) with distilled water (500 ml) for 30min (two times) at 75°C. The extract was filtered then lyophilized and it was stored at -20°C before use (The yield of extracts was 9%).
Experimental animal model and treatments
Thirty-two female Wistar rats with a weight of (65 ± 5) g were used. They were housed in the cages with four per cage and fed ad libitum. They were exposed to a 10h light:14h dark cycle and the room temperature was maintained at (24 ± 2) °C.
Animals were randomly divided into 4 experimental groups, each consisting of 8 rats:
At the end of the experiments, the rats were sacrificed by intraperitoneal injection of pentobarbital anesthesia (10%). The brains were quickly dissected out, washed with a saline solution under the ice and weighed. Then, each brain was divided into two portions (hemispheres). The right hemispheres were immediately stored at -80°C until biochemical assay. The second portion was fixed in formalin buffer (10%) for histopathological investigation.
Preparation of brain homogenate
The right hemispheres of the brain were homogenized into phosphate buffer solution (PBS, 0.1M; pH=7.4) at the rate of 1g/10ml. Rat brain homogenates were centrifuged at 3000r/min for 15 min at 4°C, the resultant supernatant was centrifuged for 10min at 10000r/min and 4°C and the final surnageant obtained was used for the estimation of antioxidant parameters and acetylcholinesterase (AChE) activity.
2.5. Behavioural parameters:
The open-field test is the most popular test of behaviors [44] for the evaluation of motor activity as a reaction to an unknown environment by exploration [45]. It is utilized to provide a quantitative and qualitative measurement of exploratory and locomotor activity in rodents.
The apparatus consists of a wooden box (90cm × 90cm × 25cm) divided into 36 equal squares (15cm × 15cm). Individual animals were placed in the center of the apparatus and allowed to freely explore the maze for 30min. The following behavioral components were measured: rearing (standing upright on the hind legs), locomotion (the total number of square crossing with all four paws), grooming (includes face cleaning, licking and scratching), and sniffing (the nose is held close to the floor).
The field must be cleaned from trial to trial using 70% ethanol and wiped dry.
The Y-maze is used to measure short term memory, and stereotypic behavior [46]. Rodents usually prefer to explore a new arm of the maze rather than returning to one that was visited previously. Spontaneous alternation behavior is a typical manifestation of short-term special (work) memory measured in the Y- labyrinth task [47].
Therefore, the Y-maze is composed of 3 equally arms (120°, 15cm high and 41cm long). Each animal was placed into the end of the start arm facing the wall and was allowed to freely explore the three arms labeled A, B and C for 5min.
The sequence of arm entries is recorded. The subject should show a tendency to enter a less recently visited arm and the percentage of alternation is calculated as ((actual alternations / maximum alternation) ×100). The apparatus was cleaned with 70% ethanol and allowed to dry between sessions.
Biochemical Estimation
The determination of catalase (CAT) activity was carried out according to the method of [48]. The mixture of the reaction, containing 0.2 ml brain homogenate, 0.2 ml phosphate buffer (1/1.5M, pH=7), and 0.2 ml H2O2 solution (0.2M) were agitated then incubated at room temperature for 5min, the reaction was stopped by the addition of 0.2 ml solution of acetic acid/potassium dichromate (3V/1V) and the absorbance was directly measured at 570nm. The results were presented as μmol hydrogen peroxide consumed/mg of protein.
Lipid peroxidation level was measured by the quantification of thiobarbituric acid-reactive substances (TBARS) according to the method of [49]. Briefly, 0.1ml brain homogenate was added to 0.1 ml sodium dodecyl sulfate (8.1%), then completing volume with 0.3ml distilled water. The mixture was incubated at 95°C for 60 min then cooled.
After incubation, 0.5 ml distilled water and 2.5 ml butanol/pyridine (15v/1v) were added, the final solution was centrifuged at 4000r/min for 10min. The absorbance of the surnageant was measured at 535nm and the amount of malondialdehyde (MDA) formed was calculated based on the molar extinction coefficient of MDA (1.56 × 105 M-1cm-1). The results were expressed as μmol of MDA/mg protein.
AchE activity was assayed according to the method of [50]. The reaction mixture contained 0.02ml brain homogenates, 3ml phosphate buffer (100 mM, pH=8.0) and 0.1 ml DTNB. The reaction was initiated by adding 0.05 ml acetylthiocholine solution. The absorbance of the complex was recorded each 2min for 10 min at 412nm at 25°C and the enzyme activity was expressed as micromoles of acetylthiocholine hydrolyzed / min/mg protein.
The protein content was estimated by the method of [51] using bovine serum albumin as the standard.
2.7. Histologie
The left hemisphere of each brain was fixed in the 10% formalin solution and embedded into paraffin. Paraffin blocks were cut at a thickness of 4 μm using the rotary microtome. Finally, the sections were stained with hematoxylin and eosin (H & E) then examined using the light microscope.
Statistical analysis
The data were expressed as mean value ± standard error. The differences between the means were analyzed with one-way analysis of variance ANOVA followed by Tukey's post hoc test using the SPSS 23 version. A level of P value less than 0.05 was considered to be significant.
RESULTS
Effect of Aluminum chloride and treatment on body weight, weight gain, and relative brain weight
Table 1: Effect of AlCl3 exposure and Salvia officinalis treatment on body weight, body weight gain and relative brain weight
|
Experimental groups |
||||
|
|
Control |
AlCl3 |
AlCl3 + SAE |
SAE |
|
Initial BW (g) |
67.49 ± 1.88 |
67.54 ± 2.15 |
67.08 ± 2.04 |
67.54 ± 1.72 |
|
Final BW (g) |
197.18 ± 6.8 |
164.97 ± 7.41 |
187.47 ± 7.48### |
197.63 ± 8.03### |
|
Body Weight gain (g) |
129.69 ± 6.36 |
97.44 ± 6.84*** |
120.4 ± 7.23### |
130.08 ± 8.26### |
|
Relative brain weight (g) |
0.99 ± 0.04 |
0.93 ± 0.04* |
0.96 ± 0.05 |
1.00 ± 0.05## |
Values are given as mean±SEM each group. ( *: P≤0.05, ***: P≤0.001) compared with the control group; (##: P≤0.01, ###: P≤0.001) compared with AlCl3 group.
The results in Table 1 show that rats intoxicated with AlCl3 induced a significant decrease (P≤0.001) in body weight and weight gain compared to the control rats. Treatment of AlCl3-induced rats with S.officinalis aqueous extract significantly (P≤0.001) increased body weight with a significant change in weight gain (23.56%) compared to the AlCl3 group.
The relative weight of the brain also significantly decreased (P≤0.05) in the AlCl3 group in comparison to the control one, treatment with S.officinalis resulted in the recovery of relative brain weight compared to the AlCl3 group.
Effect of treatment on behavioural parameters
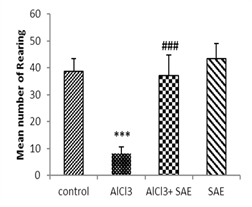
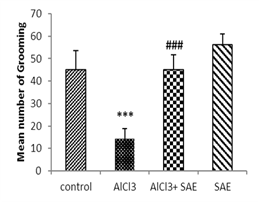
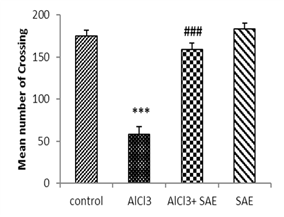
One-way ANOVA and Tukey analysis of the behavioural parameters in the open field (Fig 1) showed a significant decrease of 66.66% in crossing, 79.27% in rearing, 70.64% in sniffing, and 68.0% in grooming, while this decrease was lower in intoxicated treated rats (9.47%, 4.14%, 13.26%, and 0%, respectively) compared to the control one.
In contrast, the intoxicated rats treated with the aqueous extract of Salvia officinalis had a significant (P ≤ 0.001) increase in crossing, rearing, sniffing, and grooming compared to the AlCl3 group.
 |
 |
 |
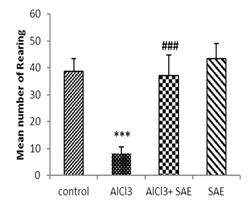 |
Figure 1: Effect of AlCl3 exposure on Open-Field parameters (crossing, rearing, sniffing, and grooming).
Values are given as mean ± SEM. ***: P≤0.001 compared to the control group, ###: P≤0.001 compared to the AlCl3 group.
The statistical analysis of the results indicated that spontaneous alternation significantly reduced (47.01%) in the AlCl3 group compared to the control, whereas the S.officinalis treatment of AlCl3-induced rats (AlCl3+SAE) significantly increased spontaneous alternation (53.05%) compared to the AlCl3 group (Fig 2).
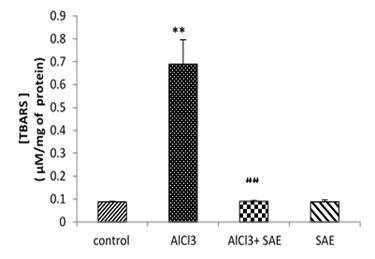
Figure 2: Effect of S.officinalis on the working memory of control and AlCl3-induced rats.
Values are given as mean ± SEM. ***: P≤0.001 compared to control group, ###: P≤0.001 compared to the AlCl3 group.
Estimation of the antioxidant enzyme level in the brain homogenate of rats
The obtained results in Fig 3 revealed that the brain MDA level significantly (P≤0.001) increased in the AlCl3 group compared to the control.
The administration of the aqueous extract of sage to rats intoxicated with AlCl3 caused a significant reduction (P≤0.001) in the elevated brain TBARS concentration (-86.81%) compared to the AlCl3 intoxicated group.
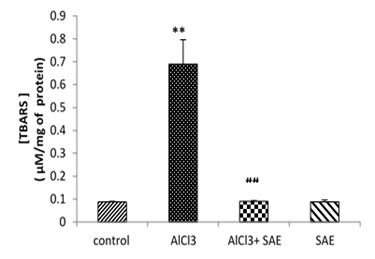
Figure 3: Effect of AlCl3 on TBARS in rat’s brains before and after treatment by Salvia officinalis.
Values are given as mean ± SEM. ***: P≤0.001 compared with the control group, ###: P≤0.001 compared with AlCl3 group.
Fig 4 shows the effect of chronic exposure to AlCl3 and treatment on Catalase activity.
AlCl3 administration induced a significant reduction (P≤0.001) of Catalase activity as compared to the control. In contrast, the treatment of AlCl3-rats with S.officinalis (AlCl3+SAE group) induced a significant elevation (P≤0.001) in CAT activity about 62.7% compared to AlCl3 group.
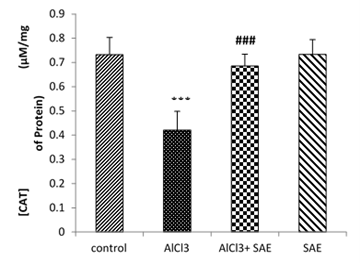
Figure 4: Effect of S.officinalis treatment on CAT activity of rats exposed to AlCl3 for 90days.
Values are given as mean ± SEM. ***: P≤0.001 compared with the control group; ###: P≤0.001 compared to the AlCl3 group
Effect of treatment on AChE activity
The statistical analysis showed that AlCl3-exposure in rats significantly decreased the level of AChE (P≤0.001, -54.49%) in comparison to the control group, but significant elevation of AChE level (73.68%) was recorded in the intoxicated treated group compared to the AlCl3 group (Fig 5).
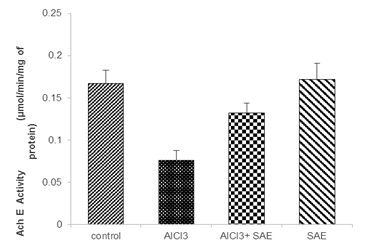
Figure 5: Effect of S.officinalis on AChE activity on the brain of control and AlCl3-exposure rats.
Values are given as mean ± SEM. ***: P≤0.001 compared to the control group, #: P≤0.05 compared to the AlCl3 group.
Effect of treatment on brain histopathological changes
Fig 6 shows the effect of Salvia officinalis aqueous extract in aluminum-induced histological changes in the brain of the experimental rats.
The microscopic examination of the brain section of the control and treated groups showed normal morphological structure (Fig 6; A and H). On the other hand, the brain section of AlCl3 animals showed marked deterioration as visualized by vacuolated cytoplasm, dilatation of blood capillary, reduced pyramidal cells in comparison to the control group (Fig 6; B, C, D, and E).
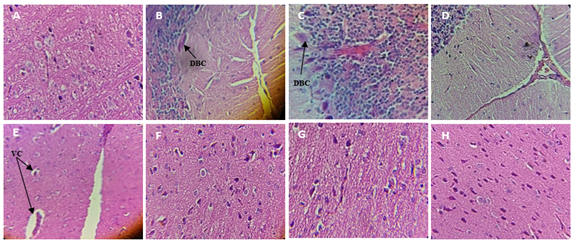
Figure 6: Effect of Salvia officinalis aqueous extract on AlCl3-induced histological changes in the brain of the experimental rats (H & E, 40×).
A (control); B, C, D, and E (AlCl3: 60mg/kg BW); F and G intoxicated treated group (AlCl3 + AE); H treated group (AE).
VC: Vacuolated Cytoplasm,
DBC: dilatation of blood capillary
DISCUSSION
The current study suggests that AlCl3 exposure caused a significant reduction in the final body, gain body weight and relative brain weight compared to the control group. These results are in agreement with previous studies such as those of Ogueche et al. [52] who observed that the bodyweight of rats exposed to aluminum decreased with an increase in the concentration of aluminum. Thus, Bharathi et al. [53] concluded that AlCl3 exposure resulted in significant weight loss. Therefore, this reduction appears to be caused by a decrease in food intake.
In this study, we investigated biochemical, behavioral, and histopathological alterations caused by chronic AlCl3 exposure and the possible effect of treatment with the aqueous extract of Salvia officinalis thas has been used traditionally to improve memory and reduce age-related cognitive decline.
The present results demonstrated that female rats exposed to AlCl3 differ from their control by their locomotor behavior, resulting in a significant decrease in the average number of a cross, reaning, sniffing, and grooming. Our results are consistent with other research showing that AlCl3 exposure affects the development and behavior of rats [54]. Besides, Erazi et al. [55] found 47% of reduction in locomotor performance in aluminum-treated rats.
These behavioral changes may result from subtle changes in serotonin levels in brain regions after AlCl3 exposure. A significant decrease in serotonin and dopamine levels was observed after treatment in the brain and cerebellum compared to the control group [54].
According to the present results, working memory was also affected by AlCl3 exposure for 3 months by recording a significant reduction of 47.01% in spontaneous alternation; it is likely that Aluminum causes a cognitive impairment characterized by a deficiency of working memory. This is consistent with different studies of memory, in which Aluminum causes cognitive dysfunction and negatively affects the spatial learning and memory capacity of rats such as Zghari et al. [56] studies who reported that chronic exposure to Aluminum induced a significant decrease of spontaneous alternation in male and female rats. Moreover, spatial memory as measured by the y-maze test depends on the learning function and memory of the hippocampus and is related to the NMDA receptor/Ca2+ influx signaling pathway [57].
Aluminum may inhibit this receptor. Many authors have demonstrated that these receptors involved in spatial learning [58], working and reference memory [59].
In our current work, the AlCl3-intoxicated rats receiving the aqueous Salvia officinalis extract exhibited behavior similar to that of the control group with a significant difference compared to the AlCl3 group. These results indicate that Salvia officinalis may be useful in the development of a therapeutic agent for neuroprotection. Thus, Eidi et al. [60] concluded the effect of Salvia officinalis leaves on memory retention and its interaction with the muscarinic and nicotinic cholinergic system in rats. Furthermore, it has been reported that aqueous extract of Salvia officinalis leaves possesses a mnemonic impact on rats in Y-maze active test [61].
The present study also showed that oxidative stress was important in the AlCl3 group, which was indicated by a statistically significant increase in the mean concentration of MDA and a significant decrease in catalase activity. These results are in agreement with previous studies; these data could be explained by an increase in lipid peroxidation of biological membranes, and alteration of the integrity of the membrane, modification of the membrane capacity, improvement of its permeability, and loss of its fluidity; affecting membrane-bound enzymes leading to cell leakage [62]. On the other hand, Ananda et al., [63] reported a significant decrease in Catalase activity in the brain of Wistar rats exposed to aluminum.
Manipulation of aluminum-intoxicated rats with Salvia officinalis extract revealed a significant reduction in MDA level and elevation in Catalase activity compared to the AlCl3 group. These results confirmed that Salvia officinalis can significantly modulate oxidative stress parameters in young female rats causing by AlCl3 through its antioxidant composition. These data are consistent with in vitro study of Miura [64], which showed that the sage is able to neutralize free radicals through the presence of many components, active agents with high antioxidant activity, such as carnosol, carnosic and rosmarinic acids, rosmadial, rosmanol, epirosmanol, methyl carnosate, and luteolin 7-O-β-glucopyranoside. These antioxidant compounds can stimulate endogenous antioxidant defense systems and trap reactive species [65]. Besides; El-kholy et al., [66] reported that aqueous Salvia officinalis extract inhibited the lipid peroxidative malondialdehyde (MDA) product in rats' brains and increase Catalase activity.
The neurotransmission induced by acetylcholine is fundamental for the functioning of the nervous system. Acetylcholine has a significant role in several aspects of cognitive function and behaviors including learning and memory. Its blockage is fatal and its progressive loss, as in Alzheimer’s disease [67], multisystemic atrophy [68] and other conditions is associated with progressive deterioration of cognitive.
The results suggest that the AlCl3-induced rats showed a significant reduction in AChE when compared to the control group. These data are consistant with previous research in the brain of rats [69, 70] suggested that intraperitoneal administration of AlCl3 to rats at a dose of 4.2mg/Kg for 28 days led to a significant decrease in AChE activity. Besides, many studies reported that aluminum modifies the properties and structure of the cell membrane, inhibiting enzymes such as acetylcholinesterase [71] causing changes and alterations in cholinergic function. Amador et al. [72] reported that aluminum interferes with cholinergic neurotransmission, glumatergic and gamma-aminobutyric [73].
Treatment with S.officinalis should moderate aluminum-induced cholinergic deficits by inhibiting the destruction of acetylcholine, which explains the significant increase in AChE activity in the intoxicated treated group of 73.68% in comparison to AlCl3 group. These results could be explained by the inhibitory capacity of Salvia officinalis, which is well documented in the literature; previous studies have suggested its possible action on acetylcholinesterase by reduction of acetylcholine degradation [74].
According to histological findings, histological changes were seen in the brain of AlCl3-exposed rats when compared to the control one. Based on these results, we, therefore, concluded that AlCl3 exposure has neurodegenerative impacts on the brain histology of rats. The pattern of changes seen in this study is similar to those of Buraimoh et al. [75] who showed that the administration of different doses of AlCl3 for 12 weeks induced neural vacuolation and cortex necrosis cerebral, reduced pyramidal cells on the brain of rats as an indication of neurodegeneration.
The powerful antioxidant activity of the aqueous extract of Salvia officinalis and its ability to increase antioxidant enzymes and promote oxygen to the brain could prevent AlCl3-induced oxidative damage. Thus, Iuvone et al. [27] demonstrated the cytoprotective effect of sage against the toxicity of Aβ neuronal cells (beta-amyloid plaques).
CONCLUSION
In conclusion, this study revealed that the treatment with the aqueous extract of Salvia officinalis in AlCl3-intoxicated rats significantly reduced the oxidative stress status and improved biochemical parameters and caused architectural disorders; this effect has been achieved through its potent anticholinesterase activity and antioxidant capacity. These results demonstrated that Salvia officinalis aqueous extract prevented aluminum chloride-induced neurotoxicity in rat brains and provided a good therapeutic approach for intervention against neurological diseases.
REFERENCES