Red and White Cabbage Extracts: Antioxidant Effects on Bovines Albumins
Faisal Ali Al Jabr 1, Mossad A Saif 2*, Ali Salman Al Zaid 1, Mohammad Ibrahim Al Homood 1, Husain Amer Al Thani 1, Ali Mohammed Al Qadheeb 1
1 Senior medical student, College of Medicine, King Faisal University, Al Hasa, Saudi Arabia.
2 Professor of Biochemistry, Biomedical Sciences Department, College of Medicine, King Faisal University, Al Hasa, Saudi Arabia.
*Email: [email protected]
ABSTRACT
Background: Reactive oxygen species (ROS) may cause extensive tissue damages in various disease conditions. It may also induce an irreversible structural and/or functional modification of proteins. Flavonoids and their derivatives are the largest group in plant polyphenols that are known to have an antioxidant effect. The aim of the present study is to evaluate the antioxidant effects of red or white cabbage on bovine serum albumin (BSA). Methods: Fresh leaves of red or white cabbage were washed with distilled water, and sliced into small pieces. Finally, the pieces were dried and extracted with 80% ethanol overnight. The antioxidant activity of cabbage extracts were studied by ferric reducing antioxidant power (FRAP) and H2O2 scavenging assays. Statistical analysis: Statistical significances were analyzed by one-way analysis of variance (ANOVA) by using software R version 2.8.1 (R Development Core Team, 2008). Significant differences (p < 0.05) are denoted by different letters. Results: Red and white cabbage extract showed a pronounced antioxidant activity. White cabbage exhibited a highest antioxidant activities compared to red cabbage extract. Conclusion: Both red and white cabbages have a high antioxidant effects. White cabbage extract had higher antioxidant activity than red cabbage extract.
Key words: Red cabbage, white cabbage, antioxidants, advanced oxidation protein products, protein carbonyl content.
INTRODUCTION
It is hypothesized that many diseases are due to oxidative stress that results from an imbalance between the formation and detoxification of pro-oxidants. Reactive oxygen species (ROS), which are produced as a by-product of electron transport in mitochondria, as a result of normal cellular metabolism and environmental factors, such as air pollutants or cigarette smoke, are highly reactive molecules -mediated damage to cellular components such as carbohydrates, nucleic acids, lipids, and proteins and alter their functions. The increase in ROS generation or decreased antioxidant availability can result in a net increase in intracellular “oxidative stress” [1].
In living systems, free radicals and ROS are constantly generated and cause extensive damage to tissues and in various disease conditions, particularly degenerative diseases, and also lead to extensive lysis [2] An alternative is the consumption of natural antioxidants from various food supplements and traditional medicines, and there is increased interest among phytotherapy researchers to use medicinal plants with antioxidant activity for protection against oxidative stress [3].
Proteins are distributed in all cellular compartments as well as forming key components of the extracellular matrix and maintaining the osmotic pressure of plasma which are sensitive to the noxious effect of the reactive oxygen species [4]. Oxidative damage to protein functions may affect the activity of enzymes, receptors and membrane transporters [5, 6]. The oxidation of amino acid residues in proteins causes modification such as deamination, carbonyl group formation and cleavage of peptide bonds. These damages produce conformational and functional alterations in proteins that lead to the fragmentation, aggregation and enhanced proteolytic digestion [7]. Plant polyphenols are known to have multifunctional properties such as reducing agents, hydrogen denoting antioxidants and singlet oxygen quenchers and flavonoids and their derivatives are the largest group of polyphenols [8, 9].
The most important property is their capacity to act as antioxidants protecting the body against ROS and may have an additive effect to the endogenous scavenging compounds [10, 11].
Brassica vegetables are consumed by people all over the world and represent an important part of human diet. Common types of these vegetables used for food preparation include different genuses of cabbage (white, green, and red), broccoli, brussels sprouts, cauliflower, and kale. They are of great interest in nutrition and medicine because of their potent protective effects on human health. Published reports link a high intake of Brassica vegetables with reduced risk of age-related chronic illnesses such as cardiovascular diseases, hepatoprotective activity in carbon tetrachloride-treated rats and several types of cancer [12-14].
In addition, red and white cabbages are largely consumed in view of their high protein content, and recent studies have pointed out that they supply the diet with, in addition to complex carbohydrates, soluble fibers, essential vitamins and metals as well as polyphenols [15] such as flavonoids, isoflavones and lignans [16].
Several studies showing controversial results of exogenous antioxidants, debating that the type, dosage and matrix of these antioxidants may be determining factors impacting the balance between beneficial and deleterious effects of these natural compounds [17-19]. There are also some proofs that they act as pro-oxidants, under certain conditions, such as high doses or the presence of metal ions. Pro-oxidants are chemicals that induce oxidative stress, either by generating ROS or by inhibiting antioxidant systems. The antioxidant or pro-oxidant activity intimately depends on their concentration. The consequences of pro-oxidant activity could be the possible damage to the biomolecules such as DNA, proteins and lipids, and the consequent cellular death [20]. The relationship between the chemical structure and the anti-oxidant/pro-oxidant activities has been the main focus of important studies in the nineteenth century.
Aim:
Oxidized protein by the effect of oxidant action of plant extract can cause a failure of protein maintenance [21-23]. Therefore, the present study would investigate the antioxidant activities of ethanolic extracts of both red and white cabbage separately. In addition, it would investigate the pro-oxidant effects of both extracts on bovine serum albumin in vitro and compare between their effects
MATERIALS AND METHODS
Chemicals
Bovine serum albumin (BSA), 2, 4-dinitrophenylhydrazine (DNPH), Ferric chloride (FeCl3), potassium ferricyanide [K3Fe3(CN)6], ascorbic acid, sodium dodecyl sulphate (SDS), were purchase from Mann Research Laboratories Inc., USA. Plants were collected from local market Al-Othaim, Hofuf, Al-Ahsa, KSA.
Methods
Plant Extracts
The leaves of red or white cabbage flowers were separated and washed with distilled water. The leaves were sliced into small pieces and oven-dried at 40oC. The dried leaves were grinding and extracted (100 gm)/1000 ml of 80% ethanolic solution. After overnight, the extract was filtered through gauze and ethanolic solution was evaporated at 50oC. After evaporation, dried samples were placed in a desiccator over calcium sulfate to remove any remaining water. The resulting dried pigments were then used for further studies. The uses of dry plants can be effective to minimize enzymatic degradation of phonetic compounds inside plant tissues [24].
I. Estimation the total antioxidant activity of both red and white cabbage extracts by Ferric Reducing Antioxidant Power (FRAP) assay
The Antioxidant activity of red or white cabbage extract was determined by Ferric Reducing Antioxidant Power (FRAP) assay according to the method described by Santiago and Valerio, 2013 [25]. The antioxidants in the cabbage extracts reduce the Fe3+ ions in Ferricyanide into ferrocyanide (Fe2+) of a blue color which was spectrophotometrically measured at λ 700 nm. The antioxidant activity of extracts is proportional to the intensity of the blue color formed that the Increase of absorbance of the reaction mixture indicates the increase in antioxidant power of the cabbage extracts. results were expressed as milligrams of ascorbic acid equivalents per milliliter of plant extract (mg AAE/ml). The results were expressed as mg of ascorbic acid equivalents (AAE) per milliliter of plant extract (mg AAE/ml).
II. Determination of hydrogen peroxide (H2O2) Scavenging activity of Cabbage extracts (Catalase-like activity)
The scavenging activity of cabbage extracts to H2O2 is evaluated by measuring the disappearance of H2O2 at a wavelength of 230 nm according to the method of Ebrahimzadeh et al, 2010 [26]. The percentage of hydrogen peroxide scavenging activity of extracts is calculated as follows:
% Scavenged H2O2=[(Ai-At)/Ai]×100
Where Ai is the absorbance of control and At is the absorbance of test. The increase of % scavenging of H2O2 indicates the increase of antioxidant activity of cabbage extract. Results are expressed as percentage of scavenging H2O2.
I. Determination of Protein-carbonyl content
Protein carbonyl content reacts with 2,4-dinitrophenylhydrazine (DNPH) forming a stable dinitrophenyl (DNP) hydrazone adducts, which can be estimated spectrophotometrically at 375 nm, as described by Levine et al, 1990 [27]. The intensity of the produced color is proportional to the carbonyl contents of the protein. The results are expressed as µmole/mg protein using a molar extinction coefficient of 22,000 M-1 Cm-1 for the DNPH derivatives.
II. Determination of Advanced Oxidation Protein Products (AOPP)
AOPP were determined spectrophotometrically at 340 nm and are expressed in chloramine units per gram of protein (µmol/g) according to the method described by Kalousová et al., 2002 [28]. AOPP level is expressed in μmol·L−1 chloramine-T equivalents. Values are given as mean ± S.D. for independent experiments.
Statistical analysis
All the results are presented as mean ± SD of 3 independent experiments. Statistical significances were analyzed by one-way analysis of variance (ANOVA) by using software R version 2.8.1 (R Development Core Team, 2008). Significant differences (p < 0.05) are denoted by different letters
RESULTS
Antioxidant activities of red & white cabbage extracts separately
Table 1: Total Antioxidant activity of red or white cabbage extracts by using FRAP assay. The results are expressed as mg of ascorbic acid equivalents (AAE) per milliliter of plant extract (mg AAE/ml).
|
Concentration of cabbage extract (mg/ml) |
White Cabbage |
Red Cabbage |
|
5 Mean + SD p |
9.53 ± 3.32 |
4.63 ± 1.59 |
|
10 Mean + SD P |
25.26 ± 9.41 |
10.17 ± 4.85 |
|
15 Mean + SD P |
103.46 ± 23.67 |
31.83 ± 9.68 |
|
20 Mean + SD P |
125.3 ± 28.56 |
80.00 ± 17.56 |
Mean + SD of three experiments for each concentration
p values of mg AAE/ml of white cabbage extract versus red cabbage extract
At the first stage, we examined the total antioxidant activity (TAA) of prepared cabbage extracts by FRAP assay. The red or cabbage extracts differed in their TAA using ascorbic acid as a standard (Table 1). The ethanolic extracts of either white or red cabbage had a significant TAA while the white cabbage extract had the highest TAA when compared to that of red cabbage extract. Their TAA was in a concentration dependent manner (Table 1).
Table 2: Determination of H2O2 Scavenging activity of red or white cabbage extracts (Catalase-like activity). Results are expressed as mean ± SD percentage of scavenging H2O2
|
Concentration of cabbage extract (mg/ml) |
White Cabbage % Scavenging of H2O2 |
Red Cabbage % Scavenging of H2O2 |
|
5 Mean + SD P |
No effect 0.0 |
No effect |
|
10 Mean + SD P |
13.17 ± 2.1 < 0.0001 |
No effect |
|
15 Mean + SD P |
27.67 ± 6.99 < 0.0001 |
3.57 ± 0.95 |
|
20 Mean + SD P |
56.6 ± 12.92 < 0.0001 |
8.6 ± 1.75 |
p values of % scavenging activity of white cabbage extract versus red cabbage extract
The ability of ethanolic cabbage extracts to scavenge H2O2 was measured spectrophotometrically. The ethanolic white cabbage extract had high ability to scavenge H2O2 than of red cabbage extract. The scavenging activity of cabbage extracts was in a concentration dependent manner (Table 2).
Assays of Po-oxidant activities of red or white Cabbage Extracts
As shown in Figures 1 & 2, both red and white cabbage extracts have an oxidative effects on BSA through increasing the levels of both protein carbonyl and AOPP contents respectively. Their effects are a concentration-dependent manner. The oxidative effect of red cabbage is significantly higher than that of white cabbage
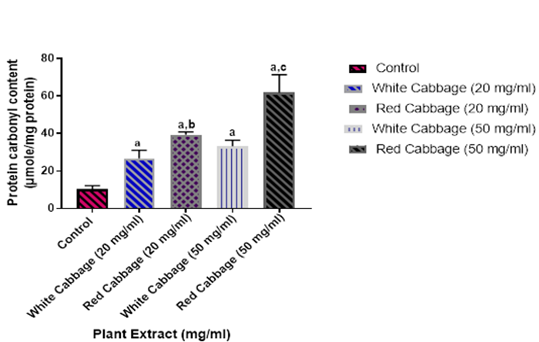
Figure 1: Effects of various concentrations from red or white cabbage extracts on the level of protein carbonyl content.
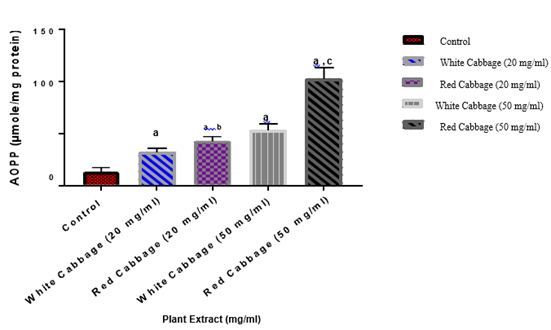
Figure 2: Effects of various concentrations from red or white cabbage extracts on the level of advanced oxidation protein products.
DISCUSSION
Natural antioxidants, including polyphenols, flavonoids, anthocyanins and carotenoids, are important part of the human diets and they represent an active principle of some medicinal plants, and play an important role in the treatment and prevention of a large number of diseases [29]. The present study revealed the antioxidant activity of the extracts of both red and white cabbages but these extracts had varied antioxidant potencies (Tables 1 & 2). These findings are confirmed by several authors who have described that phenolic compounds extracted from plants materials, have antioxidative properties in various model systems and in several foods, where they are finding increasing use [30-32]. The differences in antioxidant activities of both extracts may be due to different structures of flavonoids of these extracts and their derivatives which agree with the findings of Arouma, 2003 [33] and Kerget et al., 2005 [34].
On the other hand, the incubation of either white or red cabbage extract with BSA in vitro results in increasing of protein carbonyl and AOPP levels (Figures 1 & 2). These results can be explained to an extent in terms of the pro-oxidants activity exhibited by cabbage extracts. It has been reported that pro-oxidants are chemicals that induce oxidative stress, either by generating reactive oxygen species (ROS) or by inhibiting antioxidant systems. It has been shown that flavonoids may be unstable and thus undergo auto-oxidative reactions resulting in the production of free radicals like hydroxyl radical (.OH), superoxide radical (O⁻₂) and the semiquinone radical. Such species are known to be toxic and are reported to bind covalently and irreversibly with sulfhydryl groups and/or other essential groups of proteins producing secondary free radicals. The formation of these secondary free radicals is responsible for the pro-oxidant activity of flavonoids of cabbage extracts and depends on the concentration of the flavonoids [35, 36]. This mechanism is consistent with the study of Sang et al., 2005 [37] who showed that green tea polyphenols are unstable and undergo auto-oxidative reactions resulting in the production of ROS and the study of Santiago and Valero, 2013 [38] who found that the crude ethanolic leaf extract of F. pseudopalma stimulate hydroxyl radicals and superoxide anions. Furthermore, Gasper et al, 1994 [39] showed that quercetin gives superoxide anion by auto-oxidation which leads to the formation of H2O2 and ultimately to DNA damage. The pro-oxidant activity of cabbage extracts may also be attributed to that the flavonoid radicals (antioxidant derived radicals) formed after scavenging of primary radicals should be sufficiently stable to support an efficient antioxidant activity. The lack of stability and low redox potential of some antioxidant derived radicals may be the basis of their pro-oxidant effects toward biomolecules [40, 41].
The direct pro-oxidant properties of phenolic compounds of cabbage extracts are based on the formation of a labile aroxil radical, or a labile redox complex with a metal cation. This aroxil radical can react with oxygen, resulting in the formation of superoxide radicals (O⁻ ₂) [42].
The pro-oxidant activity of polyphenols of cabbage extracts could be also mediated by transition metals (e.g. Fe and Cu) present in biological systems through the reduction of metal ions involved in redox-cycling, and promoting the generation of hydroxyl radicals through Fenton and Fenton-like reactions. [43-45]. It is known that such metals are present as contaminants in chemical reagents, buffer, cell culture media, solvents, etc. [46]. The current study is consistent with previous studies that reported that catechol and galloyl oxidation is coupled to metal reduction (e.g., Fe3+ → Fe2+). The reduced forms of transition metals are known to catalyze lipid hydroperoxide and hydrogen peroxide decomposition to lipid alkoxyl (LO•) and hydroxyl radicals (HO•), respectively. These results suggest a role for transition metals in some of the pro-oxidant effects of green tea polyphenols observed in vitro and in vivo (Discussed below). Although the levels of transition metals are tightly regulated in vivo, the catalytic amounts necessary for the reactions describe above would indicate that such effects may still occur [47-49].
The level of phenolics, particularly anthocyanins of high antioxidant activity against ROS, was much higher in red cabbage in comparison to white cabbage [50]. The reduction of antioxidant with increasing pro-oxidant activity of red cabbage extract in the present study may be attributed to a significant reduction in phenol compounds in red cabbage with storage but this was not the case in the white [51].
In conclusion, the present study indicate that the ethanolic extracts of both red and white cabbage, which are important in the human diet, have antioxidant and pro-oxidant. The consequences of pro-oxidant activity could be the possible damage to the biomolecules such as DNA, proteins and lipids, and the consequent cellular death. In addition, the present results showed the relationship between the chemical structure and the anti-oxidant/pro-oxidant activities of food contents. Furthermore, proteins are likely to be the major targets, as a result of their abundance in cells, plasma, and most tissues, and their rapid rates of reaction both with many radicals and with other oxidants.
Data Statement:
No data were used to support this study. It is an experimental study and plants were used during the study.
REFERENCES