The Effect of Nigella Sativa, and Metformin on the Changes in Liver, Heart, and Kidney Caused by DM in Experimental Animals
Hadiya Sangi1, Nawaf M Alotaibi2, Elsamoual Ibrahim Ahmedani2, Sibghatullah Muhammad Ali Sangi2*
1 CDA Hospital, Islamabad, Pakistan
2 Faculty of Pharmacy, Northern Border University, Rafha, kingdom of Saudi Arabia.
Email: doctor_sangi @ yahoo.com
ABSTRACT
Metformin is a biguanide oral hypoglycemic drug used primarily in the treatment of DM. Nigella sativa (Active ingredient, Thymoquinone) has been found effective in lowering serum glucose levels and has protective effects preventing the complications of Diabetes Mellitus. Diabetes Mellitus causes several complications, affecting almost every organ of the body. The objective of this study was to investigate the effects of DM on liver, heart and kidney and to observe the protective as well as curative effects of Metformin and Nigella sativa from those complications. The results obtained indicate that DM causes inflammation in liver and kidney and also leads to hydropic changes. While no significant changes could be observed in the heart during the study, it was observed that Both Nigella sativa and Metformin decrease the early inflammatory changes caused by STZ induced DM in the rat liver and kidneys but the hydropic changes continue.
Key words: Diabetes Mellitus, Heart, Liver, Kidney, Complications, Nigella sativa (Thymoquinone), Metformin.
INTRODUCTION
Nigella sativa (N. sativa) (Family Ranunculaceae) is a herbal plant which is widely known with different names including black cumin, black seed and seed of blessing (Habatul-barakah in Arabic countries). The seeds have been locally used for decades in the Middle East, Far East and Asia as a food additive/spice and as effective herbal agent for health issues [1].
The seed or its oil is being used by folklore for many ailments. It is used as diuretic, lactagogue, vermifuge and condiment. It is widely used in the treatment of fever, flu, inflammation, asthma, headache, warts, and stings of scorpions and bites of snake [2-7].
Several research studies revealed that N. sativa seeds or its oil, have a broad range of pharmacological effects such as antioxidant, antimicrobial, anti-diabetic, analgesic, anti-inflammatory, anticancer, antihypertensive, anti-oxytocic, anticonvulsant, bronchodilator, diuretics, gastro-protective, hepatoprotective, immunomodulatory, pulmonary-protective, renal protective, spasmolytic and condiment [8-16].
A considerable amount of literature has been published about hepatoprotective effect of Nigella sativa by many researchers [17] that the mechanism of hepatoprotective effects of thymoquinone may be attributed to antioxidant or anti-inflammatory activities.
Metformin, is an oral hypoglycemic biguanide drug that is widely used for several decades in the treatment of type 2 diabetes mellitus. While lowering the blood glucose level, metformin can cause reduction of fat mass and inhibition of tumor cell proliferation [18, 19].
Several studies have revealed different effects of metformin on the STZ induced Diabetes mellitus in rats with improvement in the glucose and fat metabolism by influencing the level of serum total bile acids [20]. Reno protective effects of metformin against type 2 diabetes mellitus by attenuating the pathological characteristics of T2DN protecting renal functions and retaining normal morphology is very well documented [21]. In previous studies, it has been reported that metformin also possess a protective effect against the hepatotoxicity produced by STZ induced diabetes in animals [22].
MATERIALS AND METHODS
Plant Materials
Active ingredients of Nigella sativa (Thymoquinone) used in this study was purchased from the market. Metformin was acquired from the local Pharmacy.
Animals
A total number of twenty adult Wistar male albino rats, weighting 180-220g were purchased from animal unit, Faculty of pharmacy, Northern Border University. The animals were kept under standard conditions in the laboratory for two weeks for acclimatization before onset of the experiments. The study protocol was taken from the IRB, Northern Border University.
Induction of Diabetes and Experimental Design
Diabetes Mellitus was induced by a single I/P (Intraperitoneal) injection of Streptozotocin (STZ, Sigma-Aldrich), diluted in 0.1 M citrate buffer (pH 4.0), 55 mg/kg body weight [23].
The Rats were divided into four groups comprising five animals in each group as follows:
Histopathological analysis
The anesthetized rats were given a midline, longitudinal incision, starting from manubrium sterni to lower abdomen. The Skin, fascia and abdominal muscles were removed to expose abdominal viscera. After exposure, liver, Heart and Kidneys were taken out from the body and transferred to a bottle containing 10% formaldehyde solution for histological investigations.
RESULTS
Histopathological results focused mainly on three points of the organ, infiltration of the cell and interstitial changes occurring in heart, kidney and liver, over a period of time after induction of DM in experimental animals.
Histopathology of Liver:
In the control group (1), the architecture was well maintained. Liver parenchyma appeared normal with no changes in hepatocytes with normal cytoplasmic and nuclear features and normal portal triad. With regard to the structure, there were well-maintained blood vessels, intact with occasional blood vessels showing normal features. Concerning interstitium, it was intact with no changes seen (Figure 1).
Figure 1. The liver section of the negative control (H &E stain)
In the diabetic group (2), the architecture remained unchanged, parenchyma normal, hepatocytes normal cytoplasmic and nuclear features, and portal triad well-maintained. Blood vessels remained intact with congestive features. Some interstitial changes were seen with intact focal area showing aggregates of lymphocytic infiltrate (Figure 2).
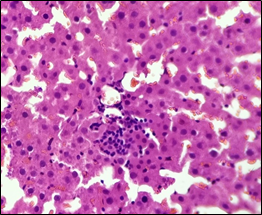
Figure 2. The section from the liver of the diabetic control group (H & E stain)
In the Diabetic group treated with Nigella Sativa (3), the architecture remained well-maintained with normal Parenchyma, normal hepatocytes with normal cytoplasmic and nuclear features and portal triad. Blood vessels were found intact with focal congestive features. Interstitium was seen intact with focal area showing sparse lymphocytic infiltrate (Figure 3).
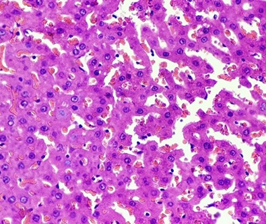
Figure 3. The diabetic liver treated with Nigella sativa (Thymoquinone) (H &E stain)
In the diabetic group treated with Metformin (4), the architecture remained well maintained, parenchyma hepatocytes with hydropic changes were found with normal nuclear features and portal triad. The blood vessels were seen congested and filled with blood. Interstitium although was seen intact with focal area showing sparse lymphocytic infiltrate (Figure 4).
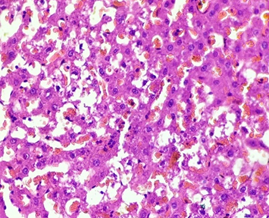
Figure 4. The diabetic liver treated with metformin (H & E stain)
Histopathology of Heart:
In the control group (1), Myocardium showed normal appearance with longitudinally-oriented cardiomyocytes, cross-striated cytoplasm and normal nucleus. Interstitium was found intact with normal features (Figure 5).
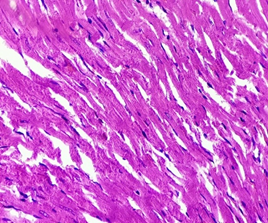
Figure 5. The section from Myocardium of the negative control group (H & E stain)
In the diabetic group (2), Myocardium showed normal appearance, longitudinally-oriented cardiomyocytes, cross-striated cytoplasm and normal nucleus. Interstitium remained intact, and no abnormality was found (Figure 6).
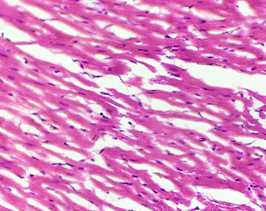
Figure 6. The section from myocardium of the diabetic group (H & E stain)
In the diabetic group treated with Nigella Sativa (3), Myocardium showed normal appearance longitudinally-oriented cardiomyocytes with cross-striated cytoplasm and normal nucleus. Interstitium remained normal (Figure 7).
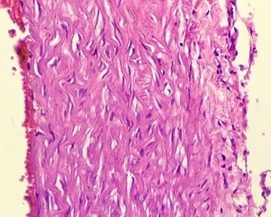
Figure 7. The section from myocardium of the diabetic group treated with Nigella sativa (Thymoquinone) (H & E stain)
In the diabetic group treated with Metformin (4), Myocardium remained normal with longitudinally-oriented cardiomyocytes, cross-striated cytoplasm and normal nucleus. No Interstitial changes were seen (Figure 8).
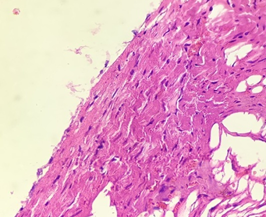
Figure 8. The section from myocardium of the diabetic group treated with metformin (H & E stain)
Histopathology of Kidney:
In the control group (1), renal architecture remained preserved. Normal glomerular cells with intact bowmen’s space, tubules intact with normal tubular epithelium and intratubular space were seen. Blood vessels were found without any changes. Intact interstitium without congestion was also seen (Figure 9).
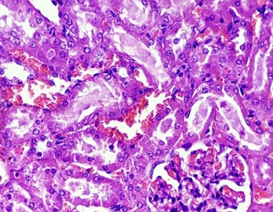
Figure 9. The section from the kidney of the control group (H & E stain)
In the diabetic group (2), the preserved renal architecture, with normal glomerular cells with intact bowmen’s space. Tubules were found intact with normal tubular epithelium and intratubular space. The blood vessels remained intact. Intact interstitium, with mild interstitial congestion was found (Figure 10).
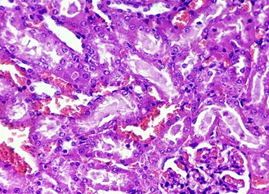
Figure 10. The section from the kidney of the diabetic group (H & E stain)
In the diabetic group treated with Nigella Sativa (3), the renal architecture remained preserved. Glomeruli remained normal with intact bowmen’s space. Tubules were seen intact with normal intratubular space and few tubular cells showing hydropic changes. The blood vessels remained intact. Little interstitial congestion was observed (Figure 11).
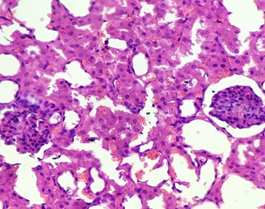
Figure 11. The section from the kidney of the diabetic group treated with Nigella sativa (Thymoquinone) (H & E stain)
In the diabetic group treated with Metformin (4), the renal architecture remained preserved. Normal Glomeruli were seen with intact bowmen’s space. Tubules were also found intact with normal tubular epithelium and intratubular space. The blood vessels were found having no changes. Intact interstitium with no interstitial congestion was seen (Figure 12).
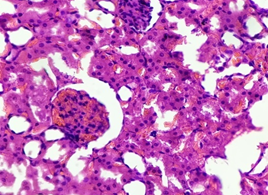
Figure 12. The section from the kidney of the diabetic group treated with Metformin (H & E stain)
DISCUSSION
Diabetes mellitus is notorious to cause damage in all the body tissues, especially in the nervous system, kidneys, blood vessels, retina, etc. As the number of patients is increasing in the world with every passing day, DM has become a global issue. It is imperative on researchers to discover a side effect-free treatment.
It is therefore necessary to study its hazardous effects in details so that a proper curative and prophylactic treatment can be found. In this study, the effects of standard drug used for the management of DM were observed in comparison to Nigella Sativa (Thymoquinone) which is having anti-hyperglycemic and anti-oxidant effects.
The results of our study show that DM causes significant damage to various organs. Even though the duration of this study was short, some damaging effects were clearly observed in the histopathological results of the study.
The aim of this study was to investigate the protective effects of Nigella sativa (thymoquinone) and Metformin on the alterations in liver, heart, and kidney caused by diabetes histologically. In order to observe this, a diabetic rat model was constituted by means of STZ injection.
This study indicates that DM mellitus caused damage to the liver and kidney even in short duration of time and initial inflammatory was observed in the architecture, and congestion/infiltration changes in interstitium. These findings were in line with the previous studies conducted [26-28].
Contrary to expectations, this research did not find any significant change in myocardial cells. The findings of the current study are consistent with the findings of the study conducted previously [29]. However conversely, in the same study, histopathology revealed a significant reduction in capillary density, which is not seen in the current study.
Nigella sativa and Metformin decreased the intensity of damage to the organs caused by Diabetes mellitus mainly due to their anti-hyperglycemic effect, and by reducing the oxidative stress. It was also observed that preventive effects of Nigella sativa active ingredient, Thymoquinone were more effective in comparison to Metformin, these results are consistent with previous results [30-36].
Many researches have concluded that diabetes induced by STZ causes biochemical effects in blood and pathophysiological differences in the liver of rats. These changes can vary from steatosis to steatohepatitis and liver fibrosis [37, 38] and that may depend on the duration of the experiment and the induction dose.
Metformin is a very well-known therapeutic agent for reduction of insulin resistance [39] and Nigella Sativa (Thymoquinone) also has been found to possess antioxidant properties [40, 41]. The preventive and curative effects of these substances from DM related damage to the cells can be attributed to the reduction in oxidative stress caused by these substances.
CONCLUSION
In the short duration, it is difficult to determine ultimate conclusion. However the results show that even in short duration of course, Diabetes Mellitus causes damage to many organs including liver. The standard drug Metformin and Nigella sativa (Thymoquinone) reduce the inflammatory changes but the damage to the cells continues especially hydropic changes. It is highly suggested that long term studies (animal and clinical) with different doses be conducted to find an effective therapeutic agent for prevention and treatment of DM-associated complications.
REFERENCES