The Study of Tha Antioxidant Response of a Biological Model Paramecium Tetraurelia in the Presence of a Nanometric Substance the Zinc Oxide
Bouchelaghem Sabrina 1*, Mouissi Samia 2, Bousaada Amina 3, Mesaadi Ibtissem 3
1Laboratory research on biodiversity and ecosystem pollution, Departement of agronomy, Faculty of Life and Natural Science, Chadli Benjedid University, El Tarf, BP 76, El Tarf, 36000. Algeria.
2 Laboratory of Agriculture and Ecosystem Functioning, Chadli Bendjedid University 36000 Algeria.
3 Faculty of Life and Natural Science, Chadli Benjedid University, El Tarf, BP 76, El Tarf, 36000. Algeria.
ABSTRACT
Zinc oxide nanoparticles (ZnO-NPs) are common nanoparticles and widely used in many fields such as sun protection products, cosmetics, pigments, industrial coatings, plastic additives, semiconductors, textiles, and antibacterial agents.
The present study was aimed to test the toxicity of ZnO-NPs on the biochemical parameters, glutathione (GSH) and catalase activity (CAT) of a single cell alternative model widely used in cellular and molecular biology: Paramecium tetraurelia.
The results obtained show that ZnO induces growth inhibition and disruption of mobility, as well as an increase in protein levels and a decrease in carbohydrate levels. Thus an increase in Catalase activity and a decrease in the level of GSH thus confirming the initiation of the detoxification process.
Key words: ZnO nanoparticles, Paramecium tetraurelia, toxicity, growth, mobility, protein, carbohydrate, GSH, CAT.
INTRODUCTION
Nanotechnology deals with the manipulation of materials at the nanoscale [1-3] The term ‘Nano’ originates from a Greek word meaning “dwarf” [4]. The nanotechnology field is evolving rapidly and is expected to disrupt all spheres of society soon. This is why several authors consider them to constitute a third industrial revolution, after mechanization and computerization. Industrialized countries therefore rightly perceive this nanoworld as carrying the immense promise of development and economic benefits. Governments, like large companies, develop strategic plans and invest colossal sums in research and development [5]. The production of nanoparticles (NPs) is increasing rapid for electronics, chemistry, and biology applications. This interest is due to the very small size of NPs which provides them with many interesting properties such as rapid diffusion, high specific surface areas, reactivity in liquid or gas phase, and size close to bio-macromolecules. However, these extreme abilities might be a problem when considering a potentially uncontrolled exposure to the environment. For instance, it is possible that nanoparticles can move around and be swifty shifted into environment or inside the body through water or air borne. Accordingly, the very fast development of these new synthetic nanomaterials raises questions about their impact on the environment and human health. [6].
This study is devoted to an in-depth understanding of the physicochemical and biological interactions between a unicellular biological model of freshwater also considered as a good bioindicator of water pollution: Paramecium tetraurelia because these cells can account for possible alterations of the environment as well. minimal as it may be [7], with ZnO nanoparticles and this through the monitoring of certain morpho-physiological parameters such as growth kinetics and markers located upstream of the cascade of events and which therefore constitute the first evidence of disturbances: total protein assays and measurement of catalase activity (CAT).
MATERIALS AND METHODS
Mixed Cultures
We infused macerate hay in a container of rainwater (drinking water is often bleach). Then, we filtered it for 24 hours, and stored the infusion (filtrate) at 15° C in a dark place, with neutral pH and well ventilated (do not cover the container). Because the cultivation and growth of the paramecium require an ambient temperature of the environment, this work had to be done for 3 months from March. The cels of Paramecium tetraurelia are grown according to the methods of Beaumont et cassier 1998, incubation is carried out in an oven at 28C±1ºC and transplanting is done every three days to have a population in the exponential phase of growth, the cell density is continuously adjusted in order obtain 104cell/ml. [8].
Growth measurement
The growth kinetics of paramecium has been carried out by measuring the optical density (ODat 600 nm (nanometer) taking the wavelength as a function of time [9]. Different treatments of zinc oxide are carried out in culture media and growth kinetics is monitored as a function of time for both witnesses of treatment.
The treatment by the oxide of the Zinc is performed after dilution in water to prepare 5 concentrations : (5,10,20,50, 100 µg/ml). The tests are performed on aliquots of 10ml of cell culture.
Determination of total protein and carbohydrate
The proteins are assayed by the method of Bradford (1976) using Albumin of Beef Serum (BSA) as standard [10]. The calibration range is carried out from a stock solution of BSA (1 mg/ml). The protein assay is done with a 100 μl aliquot. To measure the optical densities, a spectrophotometer (JENWAY 63000) is used. The measurement is carried out at a wavelength of 595 mm. Total proteins are determined from the reference curve. To determine the total carbohydrate, Duchateau & Florkin (1959) used their method which is using anthrone in sulfuric acid [11].
Determination of biomarkers
The glutathione was assayed by the method of Weckberker & Cory (1988) based on measuring the absorbance of the 2-nitro-5mercapturic resulting from the reduction of the 5-5 thiol-bis-2-nitrobenzoic acid (DTNB) by the thiol groups (-SH) glutathione [12]. We use for measuring the activity of catalase (CAT) the method of Regoli & Principato (1995). The absorbance decay is recorded for three minutes by a spectrophotometer (JENWAY 6300) for a wavelength of 240 nm and a molar extinction coefficient ε = 39400M-1 cm-1. For a final volume of 3 ml, the reaction mixture contains 100 μl of the crude enzymatic extract, 50μl of 0.3% hydrogen peroxide H2O2, and 2850 μl of phosphate buffer (50mM, pH=7.2). The calibration of the apparatus takes place in the absence of the enzymatic extract. The reaction is initiated by the addition of hydrogen peroxide. The catalase activity is expressed in nmol/min/mg of proteins.
Statistical Analysis
The statistical analysis is performed by the test (T) who is used to compare two samples (control and treated). This test is performed using the data analysis software: Statistica.
RESULTS AND DISCUSSION
The effects of oxide of the Zinc on the growth of the Paramecium tetraurelia is presented in Figure 1
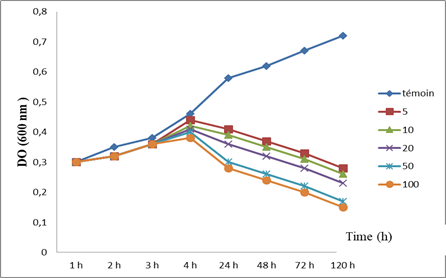
Figure 1: Effect of ZnO on the Growth of Paramecium tetrarelia.
Figure 1 illustrates the effect of ZnO-NPs on the evolution of the growth of the optical density of the paramecia, thus translating the variations in cell growth. Thus, we note that for the controls, the growth seems to increase progressively until the 4th hour when the OD is 0.46 nm to reach at the end of the treatment 0.72nm. However, for those treated with the different concentrations, growth seems to be affected. From the 24th hour of treatment, we observe a progressive decrease in the OD which will be more significant for the higher concentration (100 µg).
Effect of zinc oxide nanoparticles on total protein content in Paramecium tetraurelia.
Variations in total protein content in paramecia are shown in the Figure2
We note that this content tends to increase in a dose-dependent manner in the cells treated by the increasing concentrations of nanoparticles. this increase is very highly significant (p ≤ 0.001). Indeed, the total protein level goes from 0.0019 uM/mg of protein in the witness cells to approximately 0.031 uM/mg protein in those treated with the high concentration.
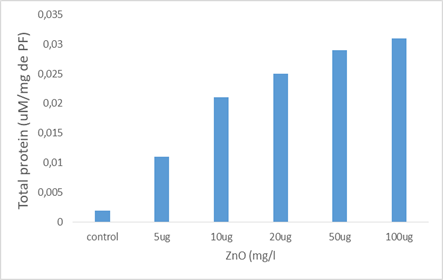
Figure 2: Effect of zinc oxide nanoparticles on total protein content in Paramecium tetraurelia.
Effect of increasing concentrations of ZnO NPs on carbohydrate levels in Paramecium tetraurelia
Figure 3 represents the variations in the carbohydrate level observed in the control paramecia and treated with two increasing concentrations of the ZnO nanoparticles.
The carbohydrate concentration of cells treated with ZnO decreases significantly (P≤0.05) in a dose-dependent manner, starting at the concentration of 20 µg / ml. For the highest concentration of ZnO (100 µg / ml), the carbohydrate level decreases by approximately 50% compared to the controls.
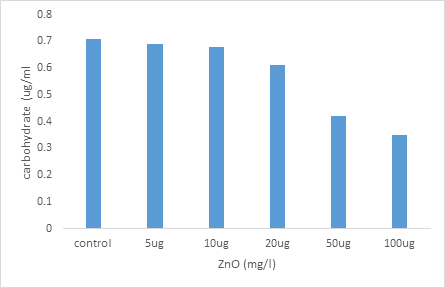
Figure 3: Effect of NPs ZnO on the carbohydrate levels in paramecium tetraurelia
Effect of increasing concentrations of NPs ZnO on the evolution of GSH levels in paramecium tetraurelia
We note that the level of GSH tends to decrease R in a dose-dependent and significant manner, particularly in the paramecia treated with the highest concentration (100 μg) compared to the controls. Thus this pass rate of 0.6 μ M / min / mg protein in controls to 0.05 .mu.M / min / mg protein in the celloules treated with the highest concentration (100 micrograms). (Figure 4).
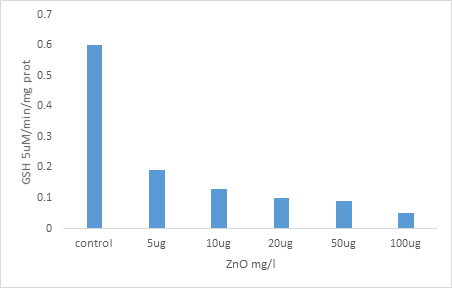
Figure 4: Effect of increasing concentrations of NPs ZnO on the evolution of GSH levels in paramecium tetraurelia
Effects of zinc oxide Nps on changes in CAT activity in Paramécium tetraurelia.
Figure 5 illustrates the evolution of CAT activity in the control paramecia and treated by increasing zinc oxide Nps concentrations. This activity tends to increase in a highly significant dependent dose (p 0.001) in cells treated with increasing concentrations of Nps compared to controls. Catalase activity increased from 0.0015 umol/min/mg protein in the control cells to 0.016 umol/min/mg protein for the highest concentration of Nps (100 ug).
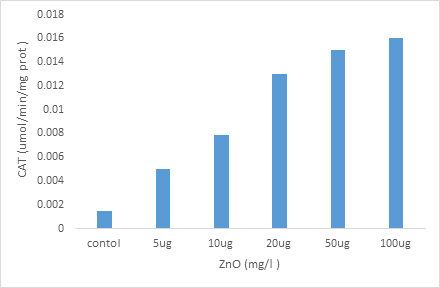
Figure 5; Effect of increasing concentrations of zinc oxide Nps on changes in CAT activity in Paramécium tetraurelia.
RESULTATS AND DISCUSSION
The bio-indicators of environmental pollution are sensitive to the physicochemical variations in their environments and also sensitive to the various xenobiotics [13], such as trace metal elements [14], or even pesticides [15]. Thus, all studies on bio-indicators converge on the idea that Paramecium is an excellent biological model for all ecotoxicological, toxicological studies and for understanding the mechanisms of action of xenobiotics [16]. The major advantage of using protozoa is, of course, the fact that they are highly specialized unicellular eukaryotes capable of performing all vital functions: moving, digesting, breathing, eliminating their waste by excretion and reproducing to survive because they have biological systems comparable to those of higher animals, such as a nucleus, vacuoles, mitochondria, a cytoskeleton, a network of membranes, etc [17]. And their responses to toxic products can be similar to those of multicellular organisms [18], which led to their use as cellular models for studying the impact of xenobiotics as well as evaluating health risks [19]. An alternative model of choice, in this case, paramecia. For this, the biomarkers evaluated relate to the concentration of GSH, total proteins, and carbohydrate as well as the Catalase activity. At the same time, the growth kinetics were also measured.
The study of the growth kinetics of Paramecium tetraurelia aims to establish a well-defined growth model highlighting with precision the different stages of a curve specific to this species of ciliated protozoa [20].
Our results show that as soon as the xenobiotic is brought into contact with the cells and the first four hours of treatment, the optical density of control and treated cells changes in a similar manner. This is in agreement with the work of [21], which demonstrated an inhibition of the growth of the paramecia during treatment with Fe3O4-NPs due to the adsorption of Fe3O4-NPs on the cell membrane and the presence of cuticle in paramecia, which makes them resistant but which nevertheless remains permeable. From 24 hours the ZnO-NPs inhibits the growth of the paramecia in a dose-dependent manner, this constitutes the first indication of the toxicity of these nanoparticles. This result is in agreement with the work of [22] or also [16] which have highlighted a disturbance in the growth of the paramecium treated with xenobiotics such as phosphoramidite. also agree with those of [23] who show that paramecia are a microorganism very close to higher organisms, that its growth is sensitive to various xenobiotics including heavy metals which makes it an excellent bioindicator of pollution [24].
During the study of the parameter of cell growth, the microscopic observation of microorganisms highlights a loss of mobility accompanied by disordered movements of the paramecia, this leads us to confirm the influx of ZnO-NPs inside cells because the cell membrane nevertheless remains permeable. This result is in agreement with [25], who have shown a reduction in the vortex movement created by the beating of the eyelashes in paramecia in the presence of high concentrations of lead, since it is known that paramecia advance by turning around its longitudinal axis, in a helical movement.
All cellular components can be affected: lipids, proteins, and therefore the membranes as a whole [26], and carbohydrates [27]. Our results highlighted a disturbance in the rate of all metabolites (increase in the total protein level and decrease in carbohydrates). These results go in the same direction as those of [16] who showed that in the paramecia treated with pesticides the rate of proteins, lipids, and carbohydrates was strongly disturbed, the author explains this phenomenon by on the one hand the induction of protein synthesis about the phenomenon of bioactivation/biotransformation, and on the other hand by the lipid peroxidation generated by ROS, this hypothesis is confirmed by the evolution of the enzymatic activities which we have followed in this part of our job. Based on the principle that any type of chemical stress can cause the release of free radicals [28], alteration of cellular components occurs when the intensity of these phenomena increases abnormally.
Glutathione is a tripeptide found in fairly high concentrations (2-10 mM) in animal and plant cells. Glutathione is found in the cytosol in high concentrations, the rest being located in the mitochondria, the nucleus, and the peroxisomes [29]. GSH deficiency exposes cells to an oxidative risk [30]. Indeed, GSH eliminates xenobiotics like pesticides by conjugation. This process may involve glutathione-S-transferase [31].
In our study we recorded a reduction in the GSH level for the different concentrations of ZnO-NPs tested as a function of time. Our results are in agreement with those of [32], who studied the toxicity of an insecticide, based on azoxystrobin and cyproconazole on Paramecium tetraurelia, where also [16], who tested phosphoramidate on Paramecium aurelia. thus and those of [5] who reported a reduction of GSH in bivalves correlated with the presence in the medium of PCB and PAH.
According to [33], glutathione plays an important role in the detoxification mechanisms of the cell and constitutes the first line of antioxidant defense. [34] confirm this role of GSH in the detoxification system.
The increase in GST activity and the decrease in GSH levels are because Glutathione is the major non-enzymatic antioxidant in Animal cells, it is the most abundant cellular thiol, involved in metabolism, Processes of transport and the protection of cells against the toxic effects of endogenous and exogenous compounds, including reactive oxygen species and heavy metals [35]. Their expression can be induced or inhibited by certain xenobiotics, which gives them great interest as potential biomarkers of pollution [36].
All of this work supports our results and confirms the role of GST, which constitutes an effective second line of defense for many highly toxic substances resulting from the interactions of reactive oxygen species with cellular macromolecules by defending the cell from the deleterious effects of stress. oxidant [37].
The catalase activity is based on the transformation of hydrogen peroxide (H2O2) into the water and molecular oxygen (O2) [38].
Our results clearly show a dose-dependent increase in this enzymatic activity in paramecia exposed to increasing concentrations of NPs-ZnO, confirming the intensification of antioxidant activity. As shown [39-41] who reported induction of CAT activity in several aquatic species.
Several researchers report very variable results concerning the activity of enzymes involved in antioxidant defense in the presence of Glyphosate; some show that this herbicide inhibits the activities of certain enzymes such as GST, SOD, acetylcholinesterase, and catalase [42] and others confirm that glyphosate causes an increase in the activity of these enzymes [43, 44]. According to [45], the use of catalase activity as an exclusive biomarker of toxicity is not recommended; Indeed, it is necessary to check the activity of other enzymes also involved in antioxidant reactions.
REFERENCES