The Effectiveness and Functional Properties of the Plant-Based Dietary Supplement in Complex Respiratory System Diseases Therapy
Pozdnyakova Olga Georgievna, Kazakova Maria Andreevna, Boisjoni Tokhiriyon* Donskova Lyudmila Aleksandrovna, Poznyakovsky Valeriy Mikhaylovich
Ural State University of Economics, Institute of Commerce, Food Technology and Service – Russia, 8 March Street 62, Yekaterinburg, 620144.
*Email: tohiriyoni @ gmail.com
ABSTRACT
The clinical trial aimed to study the effectiveness of a dietary supplement for respiratory system diseases therapy. Children underwent treatment during both acute and convalescent stages, and in addition to their primary treatment received the plant-based dietary supplement. The new plant-based dietary supplement with ingredients possessing synergetic properties was developed to supplement the normal diet and help correct metabolism disorders in the case of respiratory diseases. The ingredients for the new dietary supplement include echinacea purpurea, Vitamin C, rutin, zinc, and selenium. The consistent manufacturing process used to produce the new dietary supplement ensures that the contents are protected against adverse conditions, with the contents delivered and released at controlled rates and under specific conditions. The study of the effectiveness and the functional properties of the plant-based dietary supplement was undertaken. Children aged from 1 to 3 and treated for respiratory diseases were given ½ capsule twice a day, children aged from 3 to 7 took 1 capsule twice a day and children aged from 7 to 14 took 1 capsule three times a day as a supplement in addition to their primary medical treatment. At the same time, the children from the control group underwent only primary medical treatment. The clinical trial lasted from 10 to 14 days. During the clinical trial, regular laboratory tests and chest X-rays were carried out, and such symptoms of acute respiratory illnesses like nasal congestion, hyperaemia, sore throat, cough, wheeze as well as their duration were carefully studied. The consumption of the plant-based dietary supplement resulted in faster symptoms easing and helped relieve cough, wheeze, and nasal congestion. The data received indicates the boost to the immune response, strengthening of the immune system as a result of taking the plant-based dietary supplement. The plant-based dietary supplement is noted to be safe and it does not cause any adverse effects. The directions for use are developed. The mandatory State Registration is completed and the supplement is produced in the scientific research-to-production facilities of the Art-Life Scientific Production Association (Tomsk city).
Key words: Plant-based dietary supplement, pulmonary, clinical trial, effectiveness, functional properties, safety.
INTRODUCTION
The topic of health has always been important from the very beginning of humanity [1, 2]. The subject of health is one of the leading priorities of individuals’ lives and their satisfaction with the quality of provided health services [3]. The clinical trials are undertaken to test and evaluate the effectiveness and functional properties of specific supplements including plant-based ones. Plant-based dietary supplements are becoming increasingly popular in the prevention and adjuvant therapy of various diseases, with respiratory diseases being among them [4-11].
Almost every human being and children, in particular, suffer from respiratory diseases annually. The diseases affect patients’ immune system, weaken immune responses, and may cause the development of chronic immunodeficiency. Upper respiratory tract infections are common in both adult and pediatric populations [12].
Environmental hazards like the quality of drinking water, the safety of foods, air pollution, and others affect health and increase the risk of many illnesses. However, frequent use of chemotherapy is inadvisable, especially for children and the elderly [13-19].
Therefore, the research and development of effective and functional food supplements appear to be necessary [20].
The study aims to examine the effectiveness of using plant-based dietary supplements for respiratory system diseases and disorders therapy are undertaken when treating children during both acute and convalescent stages as well as respiratory disease prevention.
The objectives:
MATERIALS AND METHODS
The materials used plant raw materials, semi-processed materials, laboratory, and trial samples of the nutrition supplement. To evaluate the effectiveness and functional properties of the new plant-based dietary supplement 20 participants of different gender and age were chosen for the clinical trial. Ten of the children were treated at the hospital and the other ten were treated at home. The experimental group of the children treated at the hospital consisted of 8 children aged between 3 and 7, and 2 children older than 7.
The experimental group of the children treated at home included 2 children aged 1 and 3, 2 children aged 3-7, and 6 children aged 7-14. 18 out of 20 children frequently suffer from respiratory diseases, with 9 children diagnosed with acute respiratory disease, 3 children with bronchitis, 4 children with pneumonia, and 4 children with lingering symptoms of recent respiratory diseases. To confirm the diagnosis, the results of blood tests, urine tests, and chest X-rays were analyzed. The control group was composed of 20 children of similar ages and with similar diseases. All the children from the experimental (main) group received the plant-based dietary supplement together with their primary medical treatment as well as physiotherapy which is conventionally used to treat respiratory diseases.
During the clinical trial, the children from the main group aged from 1 to 3 were given ½ capsule twice a day, the children aged from 3 to 7 took 1 capsule twice a day and the children aged from 7 to 14 took 1 capsule three times a day. The duration of the clinical study was from 10 to 14 days and the dietary supplement had to be taken 15-20 minutes before eating.
RESULTS AND DISCUSSION
The clinically proven formula for the plant-based dietary supplement pellets developed to produce anti-inflammatory, immunostimulating and adaptogenic effects for mg/1 capsule weighing 0.6 gram is as follows: echinacea purpurea - 60 (oxycinnamic acids - kaftaric, chlorogenic, chicory, not less than – 1.5), Vitamin C (ascorbic acid) -60, rutin - 30, zinc asparaginate - 13.4 (zinc– 2.5), and sodium selenite - 0.014 (selenium - 0.0065).
An innovative technology to manufacture the plant-based dietary supplement in the form of pellets has been developed. To control the speed of the ingredients release and the delivery sequence, multi-layered pellets are produced.
Extensive research highlights that children with repeated respiratory diseases usually take longer to recover than children without underlying medical conditions. However, when treated with the plant-based dietary supplement, these children demonstrated positive changes and reported feeling better on the third and fourth days of the clinical trial. The swelling in their nasal passages reduced considerably, the supplement helped ease the stuffiness, relieve cough, and stop wheezing. On day 14 noticeable improvement was registered both for younger and older children from the experimental (main) group compared to the test results of the children from the control group (Fig. 1, 2).
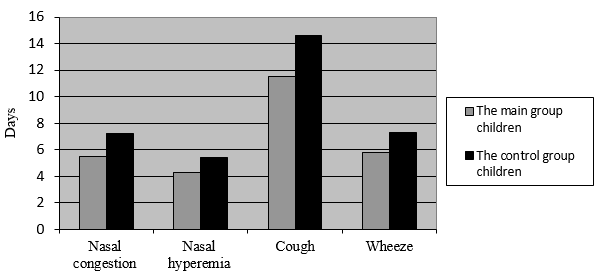
Figure 1. The Acute Respiratory Infections Symptoms Duration in days
/children aged 3-7 administered the dietary supplement/
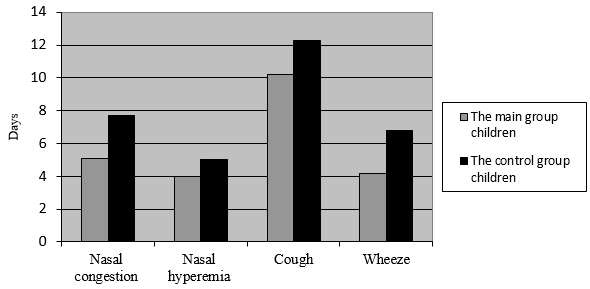
Figure 2. The Acute Respiratory Infections Symptoms Duration in days
/children aged 7-14 administered the dietary supplement/
The plant-based dietary supplement helped decrease the acute stage of the disease, lessen the severity of the symptoms, and boost immune response. The nasal congestion was cleared up 1.5 times faster. The cough was lessened by 17.21% quicker and wheezing by 21.38%, which concludes that due to the plant-based dietary supplement intake patients experience increased immune response.
The tested supplement demonstrates beneficial effects that can be achieved in complex therapy as it has stimulant properties and produces clinically proven anti-inflammatory, antioxidant, and adaptogenic effects. It should be highlighted that the children from the second (control) group who underwent conventional treatment demonstrated slower recovery and lingering asthenia.
However, the children from the experimental group experienced considerable relief and built up their strength faster, which makes it possible to recommend the plant-based dietary supplement to be used in the adjuvant therapy of acute and chronic respiratory diseases as well as in disease prevention.
When evaluating the treatment of various infectious diseases, one should take into account the major immunomodulating effects of nutritional supplements. In our plant-based dietary supplement, the main immunomodulating ingredient is Echinacea purpurea which has recently become one of the most widely used herbals in food supplements.
When evaluated, the most profound immunomodulating effect is demonstrated by polysaccharides that possess high molecular weight. Polysaccharides stimulate histogenic and hematogenous phagocytes, which ingest harmful bacteria, and macrophages, which engulf foreign substances and debris. Polysaccharides enhance immune responses and help increase the number of T-suppressor cells.
When polysaccharides and flavonoids interact, they enhance their immunomodulating effects. Together with polysaccharides, immunomodulating effects are demonstrated by chicoryic acid and other hydrophilic substances like glycoproteins and alkaloids, which trigger the activity of granulocytes and macrophages.
Therefore, Echinacea purpurea helps boost the immune response, in response to inflammatory stimuli Interleukin 1 is produced by macrophages, and B cells start secreting antibodies. Echinacea purpurea helps inhibit hyaluronidase and, thus, prevents bacteria penetration into tissues.
Considering all the above, it should be highlighted that Echinacea purpurea possesses necessary functional properties for the dietary supplement to be recommended for preventing and treating respiratory diseases. The recommended dosage is proved by the clinical trial.
To prevent diseases, ½ of the capsule should be taken. To ensure the Reference Daily Intake of the nutrients, 1 capsule should be taken daily (Table 1).
Table 1. Plant-based dietary supplement formula compared to the Reference Daily Intake
|
Ingredient |
mg |
% RDI |
|
Vitamin C |
60 |
85.7 |
|
Zinc |
2.5 |
16.7 |
|
Selenium |
6.5 |
9.3 |
|
Rutin |
30 |
100 |
|
Glycyrrhizic acid |
2 |
20 |
|
Oxycinnamic acids |
1.5 |
15 |
The plant-based dietary supplement formula and manufacturing process was developed and tested in the scientific research-to-production facilities of Art-Life Scientific Production Association (Tomsk city), and comply with ISO 9000, 9001, 22000 and GMP rules, therefore, the product stability and quality are assured.
CONCLUSION
The findings of the clinical trial indicate that the plant-based dietary supplement tested is effective both against respiratory diseases and in disease prevention and can be used in the complex therapy for children.
The children taken the plant-based dietary supplement demonstrate marked improvement; the supplement does not cause adverse effects and improves overall health.
ACKNOWLEDGEMENT
The study was performed at the premises of the Department of Therapy of the Advanced and Post-graduate Training Faculty of the Siberian State Medical University under the direction of N. V. Khudyakova, Holder of the Habilitation degree in Medicine, Professor, Honored Doctor of the Russian Federation, to whom the authors would like to express their deep gratitude.
Conflict of interest
There was no conflict of interest among the authors.
REFERENCES