The Incident of Acute Post-infectious Glomerulonephritis (PIGN) in Pediatric Age Group at King Fahad University Hospital in Eastern Province of Saudi Arabia
Hala Mohammed Al-Moaigel1, Nadeer Zaki Albaik2*, Hassan Tawfiq Al-shehab2, Abdulrahman Waheed Aldossary2, Saif Abdillah Abalkhail2, Abdulrahman Mohammed Alomani2
1 Pediatric Consultant at King Fahd University Hospital, Khobar, Saudi Arabia.
2 Medical Intern at Imam Abdulrahman Bin Faisal University, College of Medicine, Dammam, Saudi Arabia.
*Email: Nadeer.albaik @ gmail.com
ABSTRACT
Background: The aim of this study is to estimate the incidence and prognosis of PIGN in Eastern Province of Saudi Arabia. Materials and Methods: We retrospectively collected the data of all subjects who were diagnosed and got admitted with PIGN in King Fahd hospital, Al Khobar, Saudi Arabia, reviewed and approved by Institutional Review Board at Imam Abdulrahman Bin Faisal University. Result: Fifteen subjects were identified with PIGN during the study period (2009-2018). All subjects were Saudi. The mean age was 7.27 years and the majority were males (53.3%). Eleven subjects (73.3%) had recent history of URTI, eleven subjects (73.3%) had history of gross hematuria, nine subjects (60%) had history of eye puffiness, thirteen subjects (86.7%) had edema, and eight subjects (53.3%) had a fever at presentation. BP was elevated in eleven subjects (73.3%). All subjects (100%) had microscopic hematuria, urine protein to creatinine ratio was elevated in twelve subjects (median 13), ASO titer was elevated in five subjects (62.5%), C3 was decreased in twelve subjects (80%), serum albumin was low in 12 subjects (85.7%), and ANA, IgA and C4 were normal in all tested subjects. Renal biopsy was performed in two subjects and the results confirmed the diagnosis of PIGN. Conclusion: The incidence of PIGN in our pediatric population at King Fahd University Hospital in the period from 2009-2018 was 3 cases per 100000 which indicate that (PIGN) in pediatric age group in the Eastern Province of Saudi Arabia is as common as in the developed countries.
Key words: Saudi Arabia, Incidence, Prognosis, Post infectious glomerulonephritis (PIGN), Post streptococcal glomerulonephritis (PSGN).
INTRODUCTION
Post-Infectious Glomerulonephritis (PIGN) is the most common cause of acute glomerulonephritis in children. [1] It is a form of diffuse endocapillary proliferative glomerulonephritis caused by activation of the body’s immune system. The predisposing factor could be bacterial, viral or protozoal infection. The incidence in children of developing countries was 2 cases per 100,000 person-years, in contrast to 0.3 cases per 100,000 person-years in the developed countries. [2]
Children having PIGN usually present with asymptomatic microhematuria and hypertension. However, some will even progress to gross hematuria, nephrotic range proteinuria and generalized edema. PIGN usually has a good prognosis with full recovery in average 7 weeks. Minority of patients may develop complications especially if not treated.
Purpose:
There is limited data on the incidence and health outcomes of PIGN in Saudi Arabia. Our purposes of this study are:
Problem:
What is the incidence and health outcomes of Post Infectious Glomerulonephritis (PIGN) in pediatric age group presented to emergency department in King Fahad hospital of university (KFHU) over the period of 10 years (2009-2018)?
Research Background:
Definition and Causative Organism
Post Infectious Glomerulonephritis (PIGN) is the most common cause of acute glomerulonephritis in children worldwide. [1] It is characterized by an immune-mediated glomerular injury that happens as result of host reaction to non-renal infection. The commonest type of it is the post-streptococcal glomerulonephritis (PSGN), which occurs after streptococcal infection, either in the form of impetigo or pharyngitis. [3] However, there are other infectious agents that can lead to an immune-mediated glomerular changes that fall within the broad class of (PIGN) such as fungi, parasites, viruses and non- streptococcal bacteria. [3]
Incidence
It is estimated that global incidence PIGN is 472,000 cases per year, with the majority of cases in the developing countries [1]. The incidence in developing countries was 24.3 cases per 100,000 person-years in adults and 2 cases per 100,000 person-years in children, compared to 6 and 0.3 cases per 100,000 person-years respectively in the developed countries. This incidence might be under-estimated due to under-reported cases with transient symptoms [2]. In the developed countries, the incidence decreased, mostly due to early treatment of infections [4]. Aslam et al. showed that PIGN accounts for 7.5% out of all the glomerular diseases in Saudi Arabia. [5]
Pathophysiology
Circulating immune complexes play a role in the pathogenesis of PIGN. [6] Immune deposits in the glomerulus activate complement pathways and coagulation cascade and induce glomerulonephritis. In post streptococcal GN, the nephritogenic strains of group A β- hemolytic streptococcai are responsible as they have the ability to traverse the anionic Glomerular Basement Membrane to subepithelial location. [7, 8] This will be followed by in situ antibody binding. [9]
Presentation
Children having PIGN can present with asymptomatic microhematuria and hypertension or even progress to gross hematuria, nephrotic range proteinuria and generalized edema. Due to pulmonary edema, fluid overload leads to respiratory distress in severe cases. In the case of PSGN, usually the diagnosis is supported by previous history of group A β- hemolytic streptococcal infection (typically within the preceding 1 to 6 weeks depending on the source of infection). [10, 11]
Histologic patterns in PSGN
The pathognomonic histological finding in PIGN is exudative glomerulonephritis, characterized by diffuse mesangial proliferation with abundant neutrophils. Immunofluorescence microscopy with C3 staining is visible with a characteristic starry- sky pattern, corresponding to mesangial deposits and subepithelial humps on electron microscope. [12]
Management
PIGN in general is a self-limited disease, requiring mainly supportive therapy. Active infections should be treated with proper antibiotics. [3] Other symptoms should also be managed accordingly, such as hypertension, fluid overload, and electrolyte imbalances. [13]
Complication
PIGN has a good prognosis with full recovery in average 7 weeks. Minority of patients may develop complications especially if not treated. These include acute kidney failure, chronic kidney disease, end stage renal disease, rapidly proliferative glomerulonephritis, cardiac failure, pulmonary edema, hypertension, nephrotic syndrome, and hyperkalemia. [14]
Hypothesis:
Methods
In this study, we retrospectively reviewed the electronic and paper medical records of all patients ≤ 15-years-old who were diagnosed with Post-Infectious Glomerulonephritis (PIGN) and got admitted at King Fahd University Hospital in Al Khobar, Saudi Arabia between the years 2009 and 2018.
The diagnostic criteria for PIGN are microscopic or macroscopic hematuria with or without proteinuria, low serum C3 level which normalized within 8 weeks, evidence of recent streptococcal infection confirmed by throat or skin culture, or serum anti- streptomycin (ASO) of titers > 150 IU/ml. The patients with normal C3 level were included if they have fulfilled other diagnostic criteria. We also included other possible infections resulting in PIGN.
The patients with chronic kidney disease and evidence of systemic autoimmune or inflammatory diseases such as vasculitis and Systemic lupus erythematosus were excluded. Data were collected on demographics, presenting signs and symptoms, the disease that preceded PIGN, clinical course and labs tests during admission, and labs tests in the last follow-up visits.
The patients were categorized regarding their blood pressure into normotensive or prehypertensive or stage 1 or 2 hypertensive according to the percentiles adjusted for age, height and gender, and it was based on the American Academy of Pediatrics (AAP) guidelines for the pediatric hypertension revised in 2017. [15] Fever was considered when temperature was>38oC. Hematuria is defined as ≥5RBC in high power field in the urine. We assigned the patients into 4 groups, which are < 5 or 5-10 or 10-100 or > 100 per high power field. Nephrotic-range Proteinuria is considered when Urine Protein/Creatinine ratio is > 0.2. Hypoalbuminemia is defined as Serum albumin < 3.5 g/dl. BUN is considered abnormal when it is > 21 mg/dL. We considered Creatinine as high when it is > 0.7 mg/dL. Median values, means and ranges were calculated for continuous variables.
Subjects:
The patients ≤ 15-year-old who were diagnosed with Post Infectious Glomerulonephritis (PIGN) and got admitted in king Fahd hospital in Al Khobar, Saudi Arabia were included in this study.
Variables:
Independent: Not applicable
Dependent:
|
|
|
|
|
|
|
|
|
|
Controlled: Not applicable
Materials:
|
|
|
Procedures:
Results
Demographics:
A total of fifteen subjects were identified with PIGN during the 10 years study period (2009-2018). The mean age was 7.27 years ± 3.5 (ranges from 2 to 15 years). More subjects were male (53.3%). All subjects were Saudis.
Incidence:
The incidence of post infectious glomerulonephritis in our pediatric population at King Fahd Hospital of the University in the period from 2009-2018 was 3 cases per 100000.
Preceded symptoms:
Prior to the development of hematuria eleven subjects (73.3%) gave a history of recent upper respiratory tract infection, eleven subjects (73.3%) had history of gross hematuria, nine subjects (60%) had a history of eye puffiness, thirteen subjects (86.7%) had edema, eight subjects (53.3%) had a fever, three subjects (20%) had pharyngitis, two subjects (13.3%) had oliguria, and one subject (6.7%) had impetigo.
Clinical Features at Presentation:
Upon examination, blood pressure was normal in four subjects (26.7%) and abnormally high in eleven subjects (73.3%) among which stage-1 hypertension was noticed in two subjects (13.3%) and stage-2 hypertension in nine subjects (60%). Pharyngitis was seen in four subjects (26.7%), while 2 subjects had fever (13.3 %). Furthermore, edema was noticed in thirteen subjects (86.7%).
Investigations:
Hematuria was identified by microscopy in all fifteen subjects: red cells ˃ 100 per high power field (HPF) in seven subjects (46.7%), 10-100 per HPF in another seven subjects (46.7%) and 5-10 per HPF in one subject (6.7%). Renal function test (RFT) has been done to all subjects, and there was an elevation of the creatinine level and blood urea nitrogen in five subjects (33.3%). Urine Protein to Creatinine ratio was measured in twelve subjects, it was high in all of them (median 13, range 0.2 - 53.8). Throat culture was done only for nine subjects (60%). The result appeared to be positive for beta streptococcus group-A in only one subject (11.1%), while the remaining eight subjects (88.9%) were negative. ASO titer was tested in eight subjects (53.3%) and it was elevated in five subjects (62.5%) (mean 771.5 range 0 - 3697). C3 levels were decreased in twelve subjects (80%), and normal in three subjects (20%) and mean C3 was 0.51 g/L. Serum albumin was measured in fourteen subjects (93.3%) and it was low in twelve subjects (85.7%). As a part of work-ups for hematuria, IgA has been tested in eight subjects (53.3%), ANA was done to twelve subjects (80%) and C4 in all subjects and the results was normal in all of them. Renal biopsy was performed in two subjects and the results confirmed the diagnosis of PIGN.
Patients’ Management:
Regarding the patients’ management, twelve subjects (80%) were treated with diuretics. Antihypertensive medications were used for eight subjects (53.3%), only five subjects (33.3%) were treated with IV albumin, while Steroids was given to seven subjects (46.6%).
Patients Outcomes:
Thirteen subjects had follow up visits (86.6%). Mean time of follow-up visit after disease onset in weeks was 10 (ranges from 1 week to 28 weeks). Urine RBC was tested in all subjects who were following-up, four out of them had normal urine RBC, the remaining results are as shown in the tables below. Urine protein to creatinine ratio was measured in eight subjects, it was high in five subjects (75%). RFT was done for twelve subjects, and creatinine and BUN were elevated in one subjects only (6.7%). Seven subjects had their C3 level measured and all of them had attained normal values on follow up. Blood pressure measurement was documented in ten subjects, seven subjects had normal blood pressure (70%), one subject was prehypertensive (10%) and two subjects were stage 1 hypertensive (20%).
Table 1: Demographic data, signs and symptoms:
|
|
Number of Patients (percent) |
|
Saudi |
15 (100) |
|
Male |
8 (53.3) |
|
Female |
7 (46.7) |
|
Fever |
8 (53.3) |
|
URTI |
11 (73.3) |
|
Pharyngitis |
3 (20) |
|
Impetigo |
1 (6.7) |
|
Red urine |
11 (73.3) |
|
Edema |
13 (86.7) |
|
Fever |
2 (13.3) |
|
Eye puffiness |
9 (60) |
|
Oliguria |
2 (13.3) |
|
Evidence of pharyngitis |
4 (26.7) |
|
Blood Pressure Normotensive Prehypertensive Stage 1 Stage 2 |
4 (26.7) 0 2 (13.3) 9 (60) |
Table 2. The patients’ data on presentation:
|
Investigations |
Number of Patients (percent) |
Number of Patients with abnormal findings (valid percent) |
|
|
Urine RBC* |
5 – 10 10 – 100 > 100 |
15 (100) |
1 (6.7) 7 (46.7) 7 (46.7) |
|
BUN |
15 (100) |
5 (33.3) |
|
|
Creatinine |
15 (100) |
5 (33.3) |
|
|
Urine Protein/Creatinine Ratio |
12 (80) |
12 (100) |
|
|
Throat Culture positive for GAS |
9 (60) |
1 (6.7) |
|
|
ASO titer |
8 (53.3) |
5 (62.5) |
|
|
Serum Albumin |
14 (93.3) |
12 (85.7) |
|
|
C3 |
15 (100) |
12 (80) |
|
|
C4 |
15 (100) |
0 |
|
|
IgA |
13 (86.6) |
0 |
|
|
ANA |
8 (53.3) |
0 |
|
Table 3. The patients’ data on follow-up:
|
Investigations |
Number of Patients (percent) |
Number of Patients with abnormal findings (valid percent) |
|
|
Urine RBC* |
0 - 5 5 – 10 10 – 100 > 100 |
13 (86.7) |
4 (30.7) 3 (23.1) 3 (23.1) 3 (23.1) |
|
BUN |
12 (80) |
1 (8.3) |
|
|
Creatinine |
12 (80) |
1 (8.3) |
|
|
Urine Protein/Creatinine Ratio |
8 (53.3) |
6 (75) |
|
|
Serum Albumin |
10 (66.7) |
7 (70) |
|
|
C3 |
7 (46.7) |
0 |
|
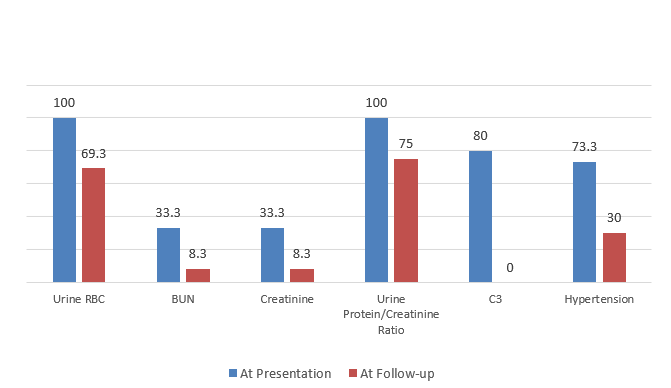
Graph 1. The percentage of abnormal clinical data at presentation and follow-up
Discussion
Incidence:
In our study, the incidence of PIGN was 3 per 100,000 which is similar with the average incidence of the developing countries whom their incidence was 2 per 100,000.
Demographics:
In our fifteen subjects, mean age was 7.27 year ranging from 2 to 15 year, which is the commonest pediatric age group to acquire the disease as found by El-Tigani et al. and Blyth et al., with an age range of 5–10 yeas. [16, 17] All of our subjects were Saudi as our hospital has eligibility for Saudi patients only. The boys affected by PIGN were higher (53.3%) compared to girls (46.7%) which is consisted with many other studies. [11, 17, 18]
Preceded symptoms:
Most of our subjects have reported recent upper respiratory tract infection (73.3%), in contrast to Blyth et al. and Becque et al. studies where almost 45% had recent upper respiratory tract infection. Three out of all URTI in our study were associated with pharyngitis (27.3%). One patient only (6.7%) had history of impetigo prior to presentation while skin diseases was more common in Becque et al. and Blyth et al. studies. [11, 17]
Clinical Features at Presentation:
We had four subjects with evidence of pharyngitis upon examination and two patients with fever which may indicate ongoing infection at presentation. The most common presentation was red urine which was presented in eleven subjects (73.3%) compared to 64% reported by Becque et al. and 44 % reported by Blyth et al. [11, 17] eleven subjects were hypertensive at presentation (73.3%) which is similar to other studies (67.6- 89%). [17, 18]
C3 levels were low in the majority of the subjects (80%), a finding that suggests PIGN upon presentation. Throat culture was positive for group A streptococcus in one subject only, and ASO titer was elevated in 62.5% of our patients, these findings supported group A streptococcal infection as the underlying cause of the PIGN. However, the remaining third of the subjects might had another infection predisposing to PIGN, yet no specific test was done to identify the organism. Renal biopsy was performed in two subjects due to the significant proteinuria at the presentation, and the results were consistent with Post Infectious Glomerulonephritis.
Patients’ Management:
The management of our subjects was mainly supportive including diuretic and antihypertensives. Furthermore, we needed to use steroids and sometimes IV albumin in some cases with severe presentation mimicking nephrotic syndrome.
Patients Outcomes:
Most of our subjects did not continue follow-up after symptomatic relief. Mean time for follow-up was 10 weeks only, and it ranged from 1 week to 28 weeks, while it is well known that complete resolution of the microscopic hematuria and hypertension might take up to one year from onset. [19] This may explain why some of the subjects still had hematuria (69.3%) and hypertension (30%) upon the follow up period. On the other hand, all subjects had their C3 levels normalized, confirming the diagnosis of PIGN. [19]
Conclusion
Post Infectious Glomerulonephritis (PIGN) in pediatric age group in the eastern province of Saudi Arabia is as common as in developing countries. Majority of the patients had complete resolution of hematuria, hypertension and renal impairment.
Application
Knowing the incidence of PIGN in our population helps us to identify patients at risk and so proper investigation.
Limitations and Recommendations
This is a retrospective study and there was difficulty in collecting the data as some of them was missed. During data collection, the hospital was shifting the paper files into electronic files and that caused some information to be lost. Pediatric nephrology services in King Fahd hospital of the university was not established till 4 years prior to conducting this study, and that might have affected the exact incidence of the disease, as many cases were referred to other hospitals. Many patients stopped follow-up after their clinical improvement, and that lead to difficulties in having an accurate prognosis for their condition. We recommend doing more studies with larger sample size to know better about the incidence and prognosis of PIGN in different parts of Saudi Arabia.
REFERENCES