The objective of this study was to document the plants used in traditional cough treatment for children and to conduct a phytochemical study of the two most used plants. An ethnobotanical survey was conducted among traditional sellers in the markets of Bamako. A phytochemical screening based on classic color reaction and tube precipitation methods was performed. Aluminum trichloride was used to quantify flavonoids, whereas the Folin-Ciocalteu method was utilized to quantify total polyphenols. The anti-protein denaturation method was used to assess the extracts' anti-inflammatory properties. In total, 56 participants, including 42 women and 14 men, were interviewed. The survey inventoried 17 antitussive plant species belonging to 14 botanical families. Sericanthe chevalieri (S. chevalieri) and Ceiba pentandra (C. pentandra) were the most frequently cited species. Phytochemical screening of these two plants revealed the presence of many major chemical groups such as alkaloids, terpenes, coumarins, tannins, saponins, and flavonoids. Macerated extracts (hydroethanolic and aqueous) exhibited the highest levels of phenolic and flavonoid compounds in both plants. The best anti-inflammatory potential, indicated by the degree of anti-protein denaturation, was observed in the hydroethanolic extracts, with an IC50 of 263.48 ± 20.80 µg/mL for S. chevalieri and 420.30 ± 19.80 µg/mL for C. pentandra. This study demonstrates that the extracts of C. pentandra and S. chevalieri are rich in bioactive substances with significant anti-inflammatory potential, which may confer antitussive properties.
INTRODUCTION
Cough is a natural and essential reflex that helps the body clear the respiratory airways [1-3]. It is the main symptom of respiratory infections or non-infectious conditions, often affecting children under the age of five. Most commonly, cough is associated with cold symptoms, particularly nasal congestion. However, it can also be triggered by other causes such as gastroesophageal reflux (GERD), bronchial inflammation (asthma, allergy), or environmental factors like tobacco smoke. In 2021, according to pediatricians, cough and cold symptoms accounted for 60% of medical consultations in Mali [4].
Antitussives used for treating cough include expectorants (guaifenesin, erdosteine, hypertonic saline solution) and agents that reduce the viscosity of secretions, such as mucolytics and mannitol [1, 5, 6]. However, alerts from the World Health Organization (WHO) regarding the safety of cough syrups, such as the 2022 case in Gambia where 66 children died, highlight the need for safer alternatives [7]. In this context, the use of medicinal plants presents an interesting and hopeful prospect [8-12].
Plants are known for their intense metabolic activity, resulting in the synthesis of a wide variety of bioactive compounds [13-18]. Many medicinal plants possess expectorant and mucolytic properties, and their use is well-documented in various cultures [19-22]. When well-studied and properly used, traditional remedies may provide an effective and safer alternative to conventional treatments. These remedies typically have fewer side effects, as they are often consumed in more natural and less processed forms. Moreover, medicinal plants are more accessible and affordable, making them especially attractive to populations in developing countries [13, 18, 23].
Additionally, these plants can not only alleviate symptoms but may also target the underlying causes of illness due to their diverse bioactive compounds [24]. It is within this context that the present study was undertaken to document the medicinal plants used in treating cough in children in Bamako, Mali. Biological investigations were conducted on the plants most recommended by traditional healers.
MATERIALS AND METHODS
Our study initially involved conducting an ethnobotanical survey among traditional healers, vendors, medical doctors, and research professors. The goal was to select plants based on citations, to carry out experimental studies in the Laboratory of Food Biochemistry and Natural Substances (LBASNa), Faculty of Sciences and Techniques (FST), at the University of Sciences, Techniques, and Technologies of Bamako (USTTB).
Zone of investigation
The survey was conducted in Bamako, specifically in the neighborhoods and markets of Lafiabougou, Hamdallaye ACI, Djikoroni ACI, and Kalaban Coura ACI. Figure 1 provides a map of the investigation areas.
|
|
|
Figure 1. Survey area map showing four neighborhoods of Bamako, Mali. |
Plant material
The plant material consisted of the bark of Ceiba pentandra (L.) Gaertn. and the leafy branches of Sericanthe chevalieri (K. Krause) Robbr., which were purchased from markets in Bamako. The identification of the studied plants was carried out at the Laboratory of Botany and Ecotoxicology of the Faculty of Sciences and Techniques (FST) at the University of Sciences, Techniques, and Technologies of Bamako (USTTB). The samples were carefully washed, dried at room temperature, then ground into powder and stored away from light and moisture.
Methods
Ethnobotanical survey
A survey form was used to document the plant species employed for treating cough in children, based on interviews conducted with market vendors and residents of the survey areas in Bamako for six months (from February to July 2022).
Study population
The study population included market vendors, traditional healers, research professors, medical doctors, and herbalists. These participants were randomly selected. The purpose of the study was explained to them to obtain their verbal or written free consent.
Preparation of extracts
Maceration was performed with 50 g of powdered plant material in 1500 mL of distilled water or 70% ethanol for 24 hours at room temperature (25–30 °C), followed by vacuum filtration. The decoction was carried out with 50 g of powdered plant material in 1500 mL of distilled water, boiled for 15 minutes at room temperature, and then filtered under vacuum.
Characterization of phytochemicals
Qualitative tube reactions were performed with the obtained extracts to identify the following phytochemical groups: polyphenols (including tannins, flavonoids, saponins, and coumarins), alkaloids, and terpenes, following the protocol described by Konaré et al. [14, 25]. The results were evaluated as follows: positive (+) and negative (-).
Total phenolic compounds assay
The quantification of phenolic compounds was performed using the Folin-Ciocalteu method, following a protocol described by Konaré et al. [14]. 500 µL of Folin-Ciocalteu reagent (diluted to 10% in distilled water) was added to 100 µL of the extract, followed by 400 µL of Sodium Carbonate (Na2CO3) at 75 mg/mL. The reaction mixture was incubated for 2 hours at room temperature, protected from light, and the absorbance was measured at 765 nm. Using the same process, a calibration curve was created using gallic acid at different concentrations (0–100 µg/mL). The calibration curve's regression line was used to calculate the amounts of phenolic chemicals present in the extracts. The results are given in milligrams of gallic acid equivalent per gram of extract (mg GAE/g). Every measurement was done three times.
Determination of total flavonoids
The estimation of total flavonoids was carried out using the aluminum chloride method, following the protocol described by Konaré et al. [14]. 200 µL of extract, 800 µL of distilled water, and 50 µL of a 5% sodium nitrite (NaNO2) solution were added, respectively. After 5 min of incubation at room temperature (25–30 °C), 50 µL of 10% (w/v) AlCl3 was added to the mixture. After re-incubation of 6 min, 400 µL of 1 M sodium acetate, and 1 mL of distilled water were also added. After homogenization, the absorbance was read at 510 nm using a spectrophotometer (Thermo Scientific, Biomate 3S). A calibration curve was obtained under the same operating conditions with quercetin at different concentrations from 20-120 µg/mL. The levels of flavonoids were deduced by linear regression equation obtained from the calibration curve and then expressed in milligrams of quercetin equivalent per gram of extract (mg QE/g).
Anti-inflammatory activity
The process of denaturing proteins was done according to Gambhire et al. [26] instructions, updated by Koné et al. [27]. To get final concentrations of 62.5, 125, 250, 500, and 1000 µg/mL, the reaction mixture (5 mL) was composed of 1 mL of egg white solution, 3 mL of phosphate-buffered saline (PBS, pH 6.4), and 1 mL of extracts at different concentrations. As the control, the same volume of distilled water was used.
The mixtures were heated to 70 °C for five minutes after being incubated for fifteen minutes at 37 °C. At 660 nm, the absorbance was measured following cooling. To determine absorbance and viscosity, sodium diclofenac was utilized as a reference molecule and treated similarly at final concentrations of 62.5, 125, 250, 500, and 1000 µg/mL. The percentage inhibition of protein denaturation was calculated using the method described by Koné et al. [27], with the equation provided below:
|
|
(1) |
Data analysis
The survey data were analyzed using SPSS software. For the statistical processing of quantitative variables (total phenolic content, total flavonoid content, and anti-inflammatory activity), Minitab v18.1 software was employed. Fisher's test with a significance level of P = 0.05 was applied in conjunction with analysis of variance (ANOVA) to compare the mean values of these variables.
RESULTS AND DISCUSSION
Ethnobotanical survey
A total of 56 people, including 42 women and 14 men, were interviewed. The respondents were aged between 25 and 75 years, with the majority being between 25 and 35 age group. The surveyed population included traditional practitioners (the majority), vendors, researchers, physicians, and herbalists. From the answers, the primary mode of administration for treating cough in children was oral, while decoction was the most common extraction method, with 86% of citations. The plants cited and their frequencies of citation are presented in Table 1.
Table 1. List and fidelity level of recommended plants
|
Scientific names |
Botanical families |
Organs used |
Fidelity Level (FL) (%) |
|
Grossopteryx febrifuga |
Rubiaceae |
Leaves + Seeds |
75.00 |
|
Anacardium occidentale |
Anacardiaceae |
Branches |
23.21 |
|
Ceiba pentandra |
Bombacaceae |
Bark |
19.64 |
|
Sericanthe chevalieri |
Rubiaceae |
Leaves |
19.64 |
|
Pteleopsis suberosa |
Combretaceae |
Branches |
17.86 |
|
Acacia albida |
Fabacea |
Bark |
12.50 |
|
Mangifera indica |
Anacardiaceae |
Leaves |
5.36 |
|
Vitex mandiensis |
Lamiaceae |
Leaves |
3.57 |
|
Piliostigma thonningii |
Fabaceae |
Leaves |
1.79 |
|
Acacia nilotica |
Fabaceae |
Leaves + Seeds |
1.79 |
|
Ximenia amercanalim |
Olacaceae |
Branches |
1.79 |
|
Vitellaria paradoxa |
Sapotaceae |
Leaves |
1.79 |
|
Saba senegalesis |
Apocynaceae |
Leaves |
1.79 |
|
Guiera senegalesis |
Guiera |
Leaves |
1.79 |
|
Pterocarpus erinaceus |
Fabaceae |
Leaves |
1.79 |
|
Ficus thonningi |
Moraceae |
Leaves |
1.79 |
Fidelity level or index (LF) is the percentage of informants who cited the use of a given species in the treatment of a pathology.
A total of 17 species were recorded. The Rubiaceae family was the most represented, followed by the Anacardiaceae family. S. chevalieri and C. pentandra were the most cited, each with a fidelity level (FL) of 19.64%. As a result, these species were selected for biochemical and biological characterizations.
Phytochemical study
The outcomes of the maceration extracts' phytochemical screening (aqueous and hydroethanolic) and the decoction of S. chevalieri and C. pentandra are summarized in Table 2.
Table 2. Phytochemical composition of S. chevalieri and C. pentandra extracts
|
|
S. chevalieri |
C. pentandra |
||||
|
phytochemical groups |
Aqueous maceration |
Hydroethanol ic maceration |
Decoction |
Aqueous maceration |
Hydroethanolic ic maceration |
Decoction |
|
Alkaloids |
+ |
+ |
+ |
+ |
+ |
+ |
|
Tannins |
+ |
+ |
+ |
+ |
+ |
+ |
|
Flavonoids |
+ |
+ |
+ |
+ |
+ |
+ |
|
Coumarins |
+ |
+ |
+ |
+ |
+ |
+ |
|
Saponines |
+ |
+ |
‑ |
+ |
+ |
‑ |
|
Terpenoids |
+ |
+ |
+ |
+ |
+ |
+ |
(+): presence et (-): absence
Table 2 highlighted the presence of various phytoconstituents such as flavonoids, tannins, coumarins, terpenoids, and alkaloids in the extracts of the sampled plants. However, saponins were absent in the decoction extracts of both plants.
Polyphenol and total flavonoid contents
The levels of specified polyphenols and flavonoids are shown in Table 3.
Table 3. Polyphenol and total flavonoid contents of S. chevalieri and C. pentandra extracts
|
Plants |
Extracts |
Flavonoids (mg QE/100g) |
Polyphenols (mg GAE/g) |
|
S. chevalieri |
Aqueous macerate |
0.268 ± 0.005aB |
0.305 ± 0.005cA |
|
Hydro-ethanol macerate |
0.125 ± 0.005cB |
0.648 ± 0.022aA |
|
|
Decocted |
0.159 ± 0.002bB |
0.431 ± 0.014bA |
|
|
C. pentandra |
Aqueous macerate |
0.468 ± 0.010aA |
0.146 ± 0.019cB |
|
Hydro-ethanol macerate |
0.070 ± 0.002cA |
0.544 ± 0.024bA |
|
|
Decocted |
0.357 ± 0.008bA |
0.350 ± 0.025bB |
*Means that do not share any lowercase letters differ greatly for each plant. The means for each extract that does not share any capital letters are notably different.
The analysis of the assays revealed that the flavonoid concentrations were higher in the aqueous extracts for both plants S. chevalieri and C. pentandra), with values of 0.268 ± 0.005 and 0.468 ± 0.010 mg QE/100 g, respectively. In contrast, the polyphenol levels were higher in the hydroethanolic maceration extracts, with values of 0.648 ± 0.022 and 0.544 ± 0.024 mg GAE/g for S. chevalieri and C. pentandra, respectively.
Anti-inflammatory activity
The results of the protein denaturation inhibition capacity with S. chevalieri and C. pentandra extracts are presented in Figures 2 and 3.
|
|
|
Figure 2. Effects of S. chevalieri extracts on protein denaturation |
|
|
|
Figure 3. Effects of C. pentandra extracts on protein denaturation |
Figures 2 and 3 demonstrated that extracts of S. chevalieri and C. pentandra could inhibit protein denaturation at concentrations ranging from 50 to 800 μ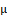 g/mL.
g/mL.
The inhibition rates of 50% protein denaturation (IC50) by extracts of S. chevalieri and C. pentandra are shown in Table 4.
Table 4. Concentrations of extracts inhibiting 50% protein (IC50)
|
|
IC50 (µg/mL) |
|
|
Extracts |
C. pentadra |
S. chevalieri |
|
Aqueous macerate |
485.60 ± 0.00a |
292.48 ± 13.97c |
|
Hydroethanolic macerate |
420.30 ± 19.80b |
263.48 ± 20.80b |
|
Decocted |
464.00 ± 18.30a |
429.12 ± 14.47a |
|
p-value |
0.006 < 0.05 |
0,00004 < 0.05 |
*For each plant species, means that do not share any letters are significantly different.
The aqueous and hydroethanolic extracts of S. chevalieri exhibited the highest inhibition capacities, with IC50 values of 292.48 ± 14 µg/mL and 263.79 ± 20 µg/mL, respectively. The extracts of C. pentandra did not show significant differences depending on the extraction method, with IC50 values of 485.62 µg/mL, 420.26 ± 20 µg/mL, and 463.98 ± 14 µg/mL for aqueous, hydroethanolic, and decoction extracts, respectively (Table 4).
Cough in children is a recurring health issue that concerns many parents. Given the risks associated with conventional medications, particularly for children, it is crucial to explore safer, cost-effective, and sustainable alternatives. In this context, the use of medicinal plants presents a promising alternative. This study aimed to catalog local medicinal plants used for treating coughs in children in Bamako. Among those surveyed, 42% were women and 12% were men. The survey identified 17 plant species across 14 families, with C. pentandra and S. chevalieri being the most notable, each with a fidelity index (NF) of 19.64%. The most represented families were Rubiaceae, Anacardiaceae, and Bombacaceae. The most used plant parts were leaves with 72 citations, followed by branches and bark with 35 and 12 citations, respectively. This preference for leaves may be attributed to their role as storage sites for secondary metabolites responsible for the medicinal properties of these plants [28-31].
The use of leaves is encouraged as it poses minimal risk to plant regeneration and contributes to the conservation of floral biodiversity [32]. The survey revealed that the most used mode of administration is oral. Romuald et al. [32] suggested that this preference for oral administration may be attributed to the fact that many of the treated conditions are associated with bacterial and fungal infections located in deeper organs [32]. Additional studies on the pharmacological properties of C. pentandra have shown that both leaves and bark are used to treat various pathologies, including cough and fever [20, 21, 33-38].
The phytochemical screening in this investigation showed that while saponins were missing from the decoctions of both plants, secondary metabolites such as alkaloids, tannins, flavonoids, coumarins, and terpenoids were present in the different extracts. These results differ from those reported by Tala et al. [38], which showed the presence of saponins in the bark of C. pentandra. This discrepancy could be attributed to methodological differences or climatic variations [39-42].
The analysis of the assays revealed higher flavonoid content in the aqueous extracts of S. chevalieri and C. pentandra (0.268 ± 0.005 and 0.468 ± 0.010 mg QE/100 g, respectively). The polyphenol content was higher in the hydroethanolic macerations (0.648 ± 0.022 and 0.544 ± 0.024 mg GAE/g for S. chevalieri and C. pentandra, respectively). This suggests that hydroethanolic and aqueous macerations are the most effective extraction methods for polyphenols and flavonoids, respectively. These findings are in line with the literature reporting the effectiveness of this solvent phenolic compounds extraction and biological activity [25, 31, 43, 44]. This richness in polyphenols and flavonoids may be responsible for their antioxidant activities and could explain their traditional use in treating inflammatory diseases [17, 21, 45, 46]. Indeed, Loganayaki et al. [36] demonstrated that extracts of C. pentandra contain high levels of polyphenols and flavonoids, which are correlated with their antioxidant properties.
Protein denaturation is one of the primary causes of inflammation [25, 26, 47]. This parameter is used to evaluate the anti-inflammatory activity of the extracts [21, 46]. Figure 2 showed that the anti-inflammatory activity was higher in the aqueous extracts of S. chevalieri. For C. pentandra, the anti-denaturation activity was similar across all extracts (Figure 3). However, the anti-inflammatory activity of the extracts from both species was lower compared to that of diclofenac. Table 4 showed that the aqueous and hydroethanolic extracts exhibited the best anti-inflammatory activities, with IC50 values of 292.48 ± 13.97 and 420.30 ± 19.80 μg/mL for S. chevalieri and C. pentandra, respectively. Statistical analyses indicated that the observed differences between the species are significant (P < 0.05). These values are lower than those reported by Abouelela et al. [20], which indicated that extracts of C. pentandra had anti-inflammatory activity comparable to ascorbic acid. This discrepancy could be due to several factors, including the plant parts used, the extraction solvents employed, and climatic variations in the area where the plants were collected [15, 48, 49]. Further analysis of the extracts of S. chevalieri and C. pentandra should also consider the chemical composition of the extracts. Previous studies have shown that flavonoids and tannins play a significant role in the anti-inflammatory activity of plants [13, 21, 50]. A more detailed characterization of the bioactive compounds present in the extracts could provide additional insights into the underlying mechanisms of the observed activity.
CONCLUSION
This study demonstrated that S. chevalieri and C. pentandra are the most used species for treating cough in Bamako, Mali. Leaves and branches are the most utilized plant parts for treating these conditions. A decoction is the most employed preparation method. Phytochemical investigation revealed that the leaves of S. chevalieri and the bark of C. pentandra are potential sources of secondary metabolites. The dosage results indicated a relatively high content of polyphenols and total flavonoids in the hydroethanolic extracts of S. chevalieri, while the aqueous extract of C. pentandra exhibited a high content of total flavonoids. Although the extracts of S. chevalieri and C. pentandra showed promising anti-inflammatory activity, these various metabolites and their anti-inflammatory effects likely explain the traditional use of these plants by local populations. However, further research is needed to optimize the characterization of active compounds and assess their efficacy in vivo. These initiatives will advance our knowledge of and ability to use medicinal plants to reduce the inflammation linked to children's coughs.
ACKNOWLEDGMENTS: We extend our gratitude to the Laboratory of Botany and Ecotoxicology, University of Sciences, Techniques, and Technologies of Bamako (USTTB), for their assistance in plant identification.
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHIC STATEMENT: None