The present study focused on lab-scale biotreatment of chromium electroplating effluent using mixed bacterial strains. The electroplating effluent-contaminated soil sample was collected and sixteen bacterial colonies were isolated and identified through morphological and biochemical characteristics. All sixteen bacterial isolates were screened for metal tolerance using a nutrient agar medium incorporated with chromium metal ions. Of the sixteen bacterial isolates, only four bacterial strains were found as potential metal-tolerant bacterial strains and they were further characterized in the various environmental conditions such as different pH, various temperatures, and different chromium metal ion concentrations. The results of the characterization study revealed that two bacterial strains, i.e., Pseudomonas sp. 4 and Staphylococcus sp. 2 were found to grow better in a medium containing 300 ppm of chromium in pH 7 at 37 °C on the 5th day. Based on molecular sequencing of 16S rRNA, the two bacterial isolates were confirmed as Pseudomonas aeruginosa and Staphylococcus caprae. Antagonistic studies between two selected bacterial strains were performed and the results indicated negative antagonistic activity between the strains. Therefore, these two compatible metal-tolerant bacterial strains were further used as a bacterial consortium for the treatment of chromium electroplating effluent in the Lab-scale reactor for 5 days. The treated effluent was collected and determined for various physicochemical characteristics through APHA, 1995 methods, and also determined the presence of the residual metal through SEM-EDAX, FTIR, and AAS analyses. Better performance metal removal was observed in the treatment with bacterial consortium.
INTRODUCTION
In general, all industrial developments result in generating wastewater in huge volumes that contains high levels of conventional as well as non-conventional contaminants which affects aesthetics and the quality of water [1, 2]. Electroplating is one of the major industrial processes that consumes and discharges large volumes of hazardous, containing heavy metals as well as cyanides, hydrogen sulfides, ammonia, and oil [3]. The major heavy metals used in the electroplating processes are copper, chromium, nickel, lead, cadmium, tin, and zinc [4].
According to Jaishankar et al. [5] and Nagajyoti et al. [6], all of these heavy metals are considered to be significant environmental contaminants, and their toxicity is an issue that is becoming more and more important for ecological, evolutionary, nutritional, and environmental reasons. The heavy metals arsenic, cadmium, chromium, copper, lead, nickel, and zinc are the most frequently detected in wastewater and are extremely dangerous to both human health and the environment [7]. More hazardous and linked to kidney impairment and lung cancer is chromium (VI). The US EPA regulates the discharge of Cr (VI) to surface waters and inland surface waters at a rate of less than 0.05 mg/L due to its toxic nature. In contrast, the total chromium, which includes Cr (III), Cr (VI), and other forms of chromium, is regulated to be discharged at a rate less than 2 mg/L [8].
However, diverse microorganisms including Bacteria are known to be resistant to heavy metals. Bacterial species possess the capacity to thrive in environments with elevated levels of metals and could also be crucial in the biological cycling of such metals, which holds immense promise for bioremediation [9, 10]. According to Dua et al. [11], microbial bioremediation is a potential biological technique that is being extensively researched for wastewater treatment. It uses microorganisms to break down or eliminate harmful waste components from the environment.
Many types of yeast, fungi, algae, bacteria, and some aquatic plants have been reported to have the capacity to concentrate metals from dilute aqueous solutions and to accumulate them inside the cell structure [12, 13]. Therefore, the biological processes are considered to be environmentally friendly, and cost-effective and enable to reduction of the operating costs and chemicals requirement for treatment. It is also effective to be applied at lower levels of contamination and acts as an alternative to conventional treatments [14, 15]. Given the above reason, the present study is focused on a study on lab-scale biotreatment of chromium-enriched electroplating effluent using an Indigenous bacterial consortium.
MATERIALS AND METHODS
Samples collection
The electroplating effluent sample used for the study was collected from the direct outlet of Vishnu Velava Bright Industry, Madurai, Tamil Nadu. The electroplating effluent-contaminated soil samples for screening of metal-tolerant bacterial strains were collected from the contaminated sites near Vishnu Velava Bright Industry, Madurai. Both effluent and soil samples were transported to the laboratory, Department of Biology, The Gandhigram Rural Institute - Deemed University, Gandhigram for further analysis.
Screening of selected bacterial isolated for chromium resistance
The sixteen predominant bacterial strains were isolated from the electroplating effluent-contaminated soil samples and were screened for their potential to tolerate chromium metal using standard procedures [16, 17]. Sixteen selected bacterial strains were inoculated on nutrient agar medium incorporated with chromium metal (100 ppm) and incubated at 37 °C for 5 days. The comparative growth performance of all bacterial isolates was observed and recorded.
Characterization of four selected heavy metal tolerant bacterial isolates
Four selected metal-tolerant bacterial strains were characterized with different conditions using standard procedures [18]. Bacillus sp. 1, Escherichia coli, Pseudomonas sp. 4, and Staphylococcus sp. 2 are four potential metal-tolerant bacterial strains that were characterized by cultivating them in a metal-based nutrient agar medium under a range of environmental conditions. These conditions included pH levels (5, 7, and 9), temperature ranges (5, 28, 37, and 45 °C), and differing concentrations of Cr (VI) metal (100, 200, 300, and 400 ppm) in various treatments for five days. The growth performance and its tolerance to chromium metal in four bacterial isolates were measured by optical density at 540 nm.
Molecular identification of metal-tolerant bacterial isolates
The two predominant metal-tolerant bacterial strains were identified through molecular sequencing using standard procedures [19]. The genomic DNA was extracted from two potential strains (Pseudomonas sp. 4 and Staphylococcus sp. 2) by using an INSTA GENETM MATRIX GENOMIC DNA ISOLATION KIT. 16S rRNA GENE amplification was carried out and it provided a 1510 base pair product for Pseudomonas sp. 4 and a 1508 base pair product for Staphylococcus sp. 2. Further, the sequences were processed for trimming at both 3’ and 5’ ends. The software “Biosystem ABI 3730xl sequencer” was used to process bacterial sequencing data. Online tool Blast N available at the National Center for Bioinformatics (NCBI), USA, was used to compare the 16S rRNA sequence of Pseudomonas sp. 4 and Staphylococcus sp. 2 with standard NCBI nucleotide database, followed by the bacterial sequence data aligned and analyzed for identifying the organisms. The NCBI Blast sequenced database for Pseudomonas sp. 4 and Staphylococcus sp. 2 showed 86% query similar with Pseudomonas aeroginosa and 95% similar with Staphylococcus caprae respectively.
Study on antagonistic activity between two metal-tolerant bacterial strains
The Antagonistic effects between two metal-tolerant bacterial strains P. aeruginosa and S. caprae were tested using a standard method [20]. A loopful of bacterial culture was taken and parallelly streaked on a nutrient agar medium containing a Petri dish. The plate was incubated at 37 °C for 24 hrs and the results were recorded.
Preparation of bacterial consortium
Based on the negative antagonistic effects, the two potential metal-tolerant bacterial strains viz., P. aeruginosa and S. caprae were selected and mass-cultured individually. Then, cultures of two bacterial strains were mixed in a 1:1 ratio carrying a fixed cell density of 1×106 cells/ml and used for the biotreatment process of chromium effluent.
Lab-scale biotreatment study on chromium effluent using mixed bacterial isolates
The biotreatment process of chromium effluent was studied with a mixed culture of two potential metal-tolerant bacterial isolates, P. aeruginosa, and S. caprae in a glass column reactor using standard procedure [21]. Effluent-based nutrient broth (500 ml) was prepared and sterilized at 121 °C at 15lbs for 20 mins. The sterile media was transferred to a 1-liter glass column and inoculated with a microbial consortium of two metal-tolerant bacterial isolates (5 ml/500 ml media with a cell density of 1×10 6 cells /ml-1). The bacterial reactor was run for 5 days. The bacterial metal effluent was collected after 5 days and the physicochemical characteristics [22], SEM- EDAX [23], FTIR [24], and AAS [25] analyses, and the results were recorded.
RESULTS AND DISCUSSION
Screening of selected bacterial isolates for chromium metal tolerance
Using morphological and biochemical traits, sixteen dominant bacterial colonies were isolated and identified. Subsequently, the genus of bacteria was determined by contrasting the features of several isolates with Bergy's Manual of Determinative Bacteriology [26]. Utilizing nutrient agar medium supplemented with 100 ppm of chromium metal ions, all sixteen of the bacterial isolates were tested for their ability to withstand chromium metal. Based on the growth performance, only four bacterial strains Bacillus sp. 1, E. coli, Pseudomonas sp. 4, and Staphylococcus sp. 2 showed better growth in comparison and were then selected for further study (Table 1).
Several authors have already reported bacterial strains capable of metal tolerant particularly bacterial strains such as Bacillus sp., E. coli, Pseudomonas sp., Staphylococcus sp., Salmonella sp., and Shigella sp. were found to be potential metal tolerant strains [27-29].
Table 1. Characteristics of sixteen bacterial isolates screened for chromium metal tolerance in culture medium with 100 ppm Cr (VI)
|
Bacterial Isolate No. |
Bacterial Strain |
Growth Performance Scale |
||||
|
Day 1 |
Day 2 |
Day 3 |
Day 4 |
Day 5 |
||
|
BIS-1 |
Pseudomonas sp. 1 |
PG |
MG |
GG |
EG |
EG |
|
BIS-2 |
Proteus sp. 1 |
NG |
PG |
MG |
GG |
EG |
|
BIS-3 |
Shigella sp. 1 |
NG |
NG |
PG |
MG |
EG |
|
BIS-4 |
Escherichia coli |
MG |
GG |
EG |
EG |
EG |
|
BIS-5 |
Proteus sp. 2 |
NG |
PG |
MG |
GG |
EG |
|
BIS-6 |
Pseudomonas sp. 2 |
NG |
PG |
MG |
GG |
GG |
|
BIS-7 |
Pseudomonas sp. 3 |
NG |
NG |
PG |
MG |
GG |
|
BIS-8 |
Shigella sp. 2 |
PG |
MG |
GG |
EG |
EG |
|
BIS-9 |
Salmonella sp. 1 |
PG |
MG |
GG |
EG |
EG |
|
BIS-10 |
Salmonella sp. 2 |
NG |
PG |
MG |
GG |
EG |
|
BIS-11 |
Pseudomonas sp. 4 |
MG |
GG |
EG |
EG |
EG |
|
BIS-12 |
Micrococcos sp. |
NG |
PG |
MG |
PG |
EG |
|
BIS-13 |
Bacillus sp. 1 |
MG |
GG |
GG |
EG |
EG |
|
BIS-14 |
Staphylococcus sp. 1 |
NG |
NG |
MG |
GG |
EG |
|
BIS-15 |
Staphylococcus sp. 2 |
GG |
EG |
EG |
EG |
EG |
|
BIS-16 |
Bacillus sp. 2 |
NG |
PG |
MG |
GG |
EG |
NG: No Growth; PG: Poor Growth; MG: Moderate Growth; GG: Good Growth; EG: Excellent Growth
Characterization of four selected chromium-tolerant bacterial isolates
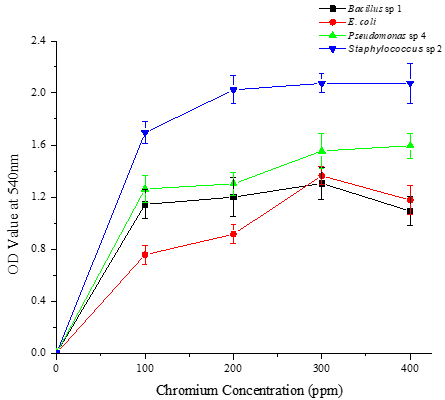
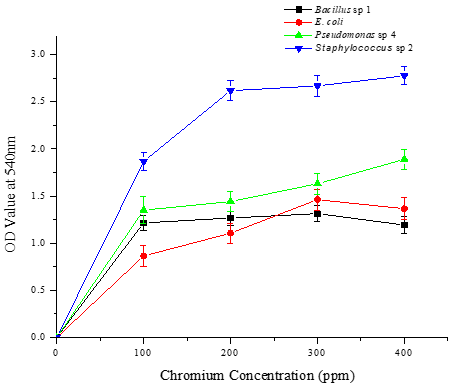
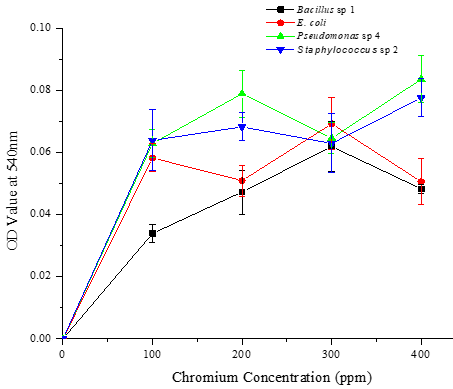
Characterized four potential chromium-tolerant bacterial strains, Bacillus sp. 1, E. coli, Pseudomonas sp. 4, and Staphylococcus sp. 2 with various growth conditions such as pH, temperature, and substrate concentrations, and the experimental results reveal that two bacterial strains i.e., Pseudomonas sp. 4 and Staphylococcus sp. 2 grown well on 400 ppm of chromium-containing nutrient broth medium with pH 7 at 37 °C (Figures 1-3). These two bacterial strains can tolerate higher concentrations of chromium in the log phase.
Six possible chrome metal-resistant bacterial strains have already been reported by Ranjithkumar and Mahalingam [30]. These strains were identified by cultivating them in a nutrient agar medium based on chromium under varied environmental conditions. In a similar vein, bacterial strains that were discovered and identified by Mehta and Vaidya [27] demonstrated chromium resistance. The ability of the bacterial strains to withstand and grow at various chromium (VI) concentrations was also reported by Tharannum et al. [31]. The bacterial strains showed optimal growth at high chromium (VI) concentrations, which they can tolerate up to 500 mg/L.
In another study, sixty-eight morphologically distinct Cr6+ resistant bacterial strains were isolated and their tolerance limit was determined. Of the 66 strains only four isolates have been found potentially tolerant to elevated chromium concentration [32]. In a previous study, Micrococcus sp. at pH 7.0 removed 90% of the chromium. Additionally, it has been found that B. licheniformis bioaccumulated lead at a rate of 0 to 1.1 mol metal/g biomass. Tripathi (2011) reported that B. cereus was able to bioremediate 74.5% Cr (VI) in 48 hours.
|
|
 |
|
a) |
b) |
 |
 |
|
c) |
d) |
|
Figure 1. Growth performance of four different bacterial strains in nutrient broth supplemented with different concentrations of chromium (100, 200, 300, and 400 ppm) in pH 5 at various temperatures (5, 28, 37, and 45 °C) on the 5th day. a) 5 °C, b) 28 °C, c) 37 °C, and d) 45 °C |
|
|
|
|
|
a) |
b) |
|
Figure 2. SEM image of chromium-tolerant bacterial strains. a) Pseudomonas aeruginosa, and b) Staphylococcus caprae |
|
|
|
|
|
a) |
b) |
|
Figure 3. SEM image of chromium electroplating effluent. a) Untreated effluent, and b) Bacterial treated effluent |
|
Study on antagonistic activity between two chromium-tolerant bacterial isolates
The Antagonistic effects between two chromium metal-tolerant bacterial strains were tested and the results revealed that the zone of inhibition was not found between P. aeroginosa and S. caprae. Elhartit et al. also reported on studying the antagonistic activity through the zone of inhibition between the different isolates of Bacillaceae and Pseudomonadaseae family against pathogenic fungi [33, 34].
Lab-scale biotreatment study on chromium effluent using mixed bacterial isolates
The biotreatment process of Chromium effluent was studied with a mixed culture of two potential metal-tolerant bacterial isolates, P. aeroginosa and S. caprae in a Lab-scale glass column reactor. The bacterial metal effluent was collected after 5 days and determined the physicochemical characteristics, SEM-EDX, FTIR, and AAS analyses (Table 2, Figures 4 and 5).
Table 2. Physico-chemical characteristics of chromium electroplating effluent before and after the bacterial treatment
|
S.No |
Physico-Chemical Parameters |
Untreated |
Bacterial Treated |
||||
|
1. |
Temperature (°C) |
31.3 |
± |
0.9 |
30.5 |
± |
0.6 |
|
2. |
pH |
3.7 |
± |
0.3 |
31.2 |
± |
3.0 |
|
3. |
Total Suspended solids (mg/l) |
1047. 7 |
± |
10.6 |
1028 |
± |
5.8 |
|
4. |
Total dissolved solids (mg/l) |
8663.3 |
± |
11.6 |
11901 |
± |
28.1 |
|
5. |
Hardness (mg/l) |
18.4 |
± |
0.6 |
10638 |
± |
13.1 |
|
6. |
Chloride (mg/l) |
266.7 |
± |
0.4 |
15.3 |
± |
3.8 |
|
7. |
Calcium (mg/l) |
126.1 |
± |
0.9 |
11.25 |
± |
0.6 |
|
8. |
Sodium (mg/l) |
148.6 |
± |
0.5 |
24.9 |
± |
0.5 |
|
9. |
Potassium (mg/l) |
218.3 |
± |
0.6 |
42.2 |
± |
10.3 |
|
10. |
Dissolved oxygen (mg/l) |
95.3 |
± |
8.0 |
1783.3 |
± |
8.3 |
|
11. |
Biological oxygen demand (mg/l) |
410.3 |
± |
8.5 |
66.3 |
± |
6.4 |
|
12. |
Chemical oxygen demand (mg/l) |
1066.0 |
± |
10.8 |
420.2 |
± |
13.2 |
|
13. |
Chromium (ppm) as per AAS |
17.5326 |
± |
0.3677 |
13.5564 |
± |
0.0007 |
(Values are mean of three replicates ± standard error)
|
|
|
|
a) |
b) |
|
Figure 4. EDAX image of chromium effluent using bacterial consortium. a) Untreated effluent, and b) Bacterial-treated effluent |
|
 |
 |
|
a) |
b) |
|
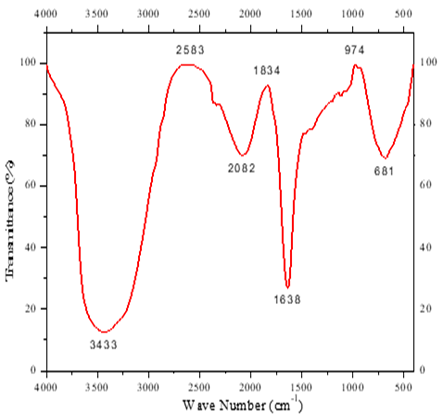
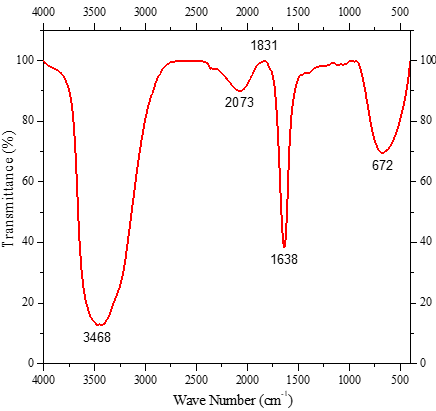
Figure 5. FT-IR Spectra of chromium electroplating effluent sample. a) Untreated effluent, and b) Bacterial-treated effluent |
|
In this study, SEM and Energy Dispersive X-ray analysis (EDX) were used to quantify the amount of chromium present in the bacterial strains P. aeroginosa and S. caprae as well as to evaluate morphological alterations in response to chromium accumulation. After five days of incubation without exposure to chromium, SEM analyses of two bacterial strains, P. aeroginosa and S. caprae, were displayed (Figure 4).
These results are in agreement with the SEM analysis of Cr (VI) treated with P.aeroginosa cell by Chatterjee et al. [35]. Their findings from SEM investigations show that before Cr (VI) biosorption, the cells seemed plump with smooth surfaces in a loosely bound state. P. aeruginosa treated with Cr (VI) develops a rough, protruding, and uneven cell surface. Since X-ray absorption provides information on the electronic and structural state of an element, EDX was used to confirm the sorption products on the surface of the bacterial cell.
Ramrakhiani et al. [36] recently reported on their SEM-EDX analysis of Cr (VI) biosorption. They collected metal SEM micrographs and EDX spectra for the metals both before and after biosorption onto the biomass. The micrographs showed that the surface of T. clypeatus cells loaded with metal clearly showed the existence of new shiny bulky particles. EDX analysis, which identified each metal peak(s) in the spectra, further supported this observation [37].
In this study, the results of FTIR spectra before and after Cr (VI) bioremediation are shown in Figure 5 where the spectra range of 4000–400 cm−1 is to determine the interaction of the metal ions and to identify functional groups which are responsible for the Cr (VI) bioremediation process. Broad spectra bands were observed at 3433 cm-1 as well as at 3468 cm−1 indicating the presence of N-H stretch. The N=C=S stretching and the C=C=S stretching were observed at 2082 cm−1, and 2073 cm−1, respectively. The changes in peaks observed between 1831 cm-1 and 1638 cm-1 could be due to the bending vibration of C-H group C=C in the remediation of Cr (VI). The band at 681.76 cm-1 was shifted to 671.91 cm-1 on Cr (VI) loaded biomass and corresponds to the stretching bond of the C =O group. Another change in the spectrum 2583 cm−1 was not obtained after Cr (VI) bioremediation. In this study, the N-H stretching group is mainly involved in Cr (VI) metal bioremediation. Peaks in the region of lower wave numbers (< 800 cm−1) can be assigned to the bending of aromatic compounds.
Furthermore, the concentration of chromium in electroplating effluent was determined before and after the bacterial treatment by an Atomic Absorption Spectrophotometer (AAS) with flame atomization. The results revealed that the concentration of chromium was decreased from 17.7326 ppm to 17.7092 ppm (Table 2). A similar study was undertaken by Dermentzis et al. [25] and they noted that the metal uptake was determined based on the difference between the primary and secondary concentrations. Further, it was also noted in different studies, that the uptakes of Cd optimum 87 mg/g for Sargassum vulgare, 80 mg Cd/g for S. fluitans, and 74 mg/g for S. filipendula. Uptakes of Cu at pH 4.5 were qmax = 59 mg/g for S. vulgare 56 mgCu/g for S. filipendula and 51 mg Cu/g for S. fluitans [38]. Also, Kaewchai and Prasertsan [39] studied the Ni and Cd adsorption by dried cells of E. agglomerans SM 38 and found that at optimum pH their removal reached 25.2% and 32%, respectively.
Based on this study, it was suggested that the two metal-tolerant bacterial strains, P. aeruginosa and S. caprae would be used as bacterial consortium for bioremediation studies.
CONCLUSION
The extensive study on Lab-scale biotreatment of Chromium-enriched electroplating effluent using bacterial consortium results in the identification of two potential Cr6+ tolerant bacterial strains and would used as an effective bacterial consortium for the biotreatment of chromium metal contaminated effluents before discharging into the open field so as so to conserve the environment and the nature.
ACKNOWLEDGMENTS: The authors wish to thank the Vishnu Velava Bright Industry, Madurai, Tamil Nadu, India for supporting this study. The authors wish to thank the Department of Biology, GRI-DU, Gandhigram, Dindigul for providing the necessary lab facilities for the successful completion of this study. Also, the authors wish to thank the Centre for Nanoscience and Technology, GRI-DU, Gandhigram for providing the SEM-EDAX facility, Lady Dock College, Madurai for providing the AAS facility, and St. Joseph’s College, Trichy for providing the FTIR facility.
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHICS STATEMENT: None