The present study aimed to design and synthesize functionalized 2,4-diamino-1,3,5-triazines, potential inhibitors involved in immune and inflammatory responses. A two-step synthesis method, initially using a microwave reactor for the preparation of reaction intermediates, allowed us to synthesize eleven 6-aryl-2,4-diamino-1,3,5-triazines 5a-k. The intermediates were biguanide derivatives 3a-f which were synthesized under microwave irradiation for 10 min at 130 °C. The 2,4-diamino-1,3,5-triazine derivatives (5a-k) were obtained with yields from 16-86%. The compounds synthesized were submitted to biological evaluation on twelve protein kinases, through the implementation of a dose-response method which allowed the determination of median inhibitory concentration or IC50. Indeed, enzymatic activities were carried out in the presence of 10 µM of ATP, in a final volume of 6 µl for 30 min at 30 °C in ADP-Glo buffer. Among the triazines synthesized, results of enzymatic activity showed that the most active molecule was compound 5b which inhibited PIM1 kinase with IC50 = 1.18 µg/mL. This result is the starting point of a larger research program for our group which could investigate the introduction of the substituted group on triazine’s terminal amino group.
INTRODUCTION
Protein kinases (PK) have been shown to play a central role in cell survival and regulatory processes, mainly at the level of proliferation mechanisms and immune response [1-5]. As a result, they constituted a set of increasingly sought-after targets in the treatment of cancers, and inflammatory and autoimmune diseases [6-12]. Thus, as part of the development of new so-called targeted therapies, we carried out the design and synthesis, in two steps, of new compounds with 2,4-diamino-1,3,5-triazine moiety. The literature described several routes to access these compounds [13-15] from arylamine or aryl nitrile derivatives with some ester derivates using a microwave reactor [16, 17]. The present study aimed to design and synthesize functionalized 2,4-diamino-1,3,5-triazines, potential inhibitors involved in immune and inflammatory responses. We reported here the results of syntheses and biological evaluation of these compounds on several protein kinases.
All commercial reagents were used for our syntheses without any further purification. All solvents were reagent or HPLC grade. Analytical TLC was performed on silica gel 60 F254 plates. Column chromatography was carried out on silica gel Merck 60 (70-230 mesh ASTM) and flash Chromatography Grace Reveleris X2TM. Melting points were determined on the electrothermal IA 9000 melting point apparatus and are uncorrected. 1H and 13C NMR spectra were recorded in CDCl3 or DMSO-d6 using a Bruker AVANCE 400 MHz spectrometer. Chemical shift values were reported in parts per million (ppm) and coupling constants (J) were given in hertz (Hz). Multiplicities were reported as follows: br s = broad singlet, s = singlet, d = doublet, t = triplet, q = quartet, and m = multiplet. LC-MS analyses were run on Waters ACQUITY UPLC-MS system composed of a single quadrupole detector (SQD), mass spectrometer (MS) equipped with an electrospray ionization interface (ESI), and a photodiode array detector (PDA).
General procedure for the synthesis of biguanides
To a solution of dicyandiamide (252 mg, 3 mmol, 1 eq) in 5 mL of acetonitrile, was added the corresponding amine derivative (1 eq). Trimethylsilyl chloride or TMSCl (1.1 eq) was added dropwise to the reaction mixture. Stirring was maintained for a few min to obtain the most homogeneous mixture. This mixture, contained in a borosilicate glass vial of 35 ml equipped with snap caps, was put in a CEM Discover SP single-mode microwave reactor for 10 min at 130 °C, under pressure of 18 bar and power of 60 Watt. After 10 min, the reaction mixture was cooled in ice crushed and the precipitate formed was filtered, washed with acetonitrile or isopropanol, and dried. The biguanide derivatives (3a-f) were obtained as powders and were used with further purification for the next step of synthesis.
4-methylthio-phenyl-biguanide (3a)
Compound 3a was obtained according to the general procedure, as a purple powder from dicyandiamide 2 (252 mg, 3 mmol), 4-(methylthio) aniline 1a (0.373 mL, 3 mmol), and TMSCl (0.417 mL, 3.3 mmol).
4-Ethymorpholinyl-biguanide (3b)
Compound 3b was obtained according to the general procedure, as a coral powder from dicyandiamide 2 (504 mg, 6 mmol), N-ethyl-amino-morpholine 1b (0.8 mL, 6 mmol), and TMSCl (0.834 mL, 6.6 mmol).
4-Cyano-phenyl-biguanide (3c)
Compound 3c was obtained according to the general procedure, as an orange powder from dicyandiamide 2 (504 mg, 6 mmol), 4-aminobenzonitrile 1c (708.84 mg, 6 mmol), and TMSCl (0.834 mL, 6.6 mmol).
Benzo[d][1,3]dioxol-5-biguanide (3d)
Compound 3d was obtained according to the general procedure, as a purple powder from dicyandiamide 2 (613 mg, 7.29 mmol), 3,4-(methylenedioxy)aniline 1d (1g, 7.29 mmol), and TMSCl (1.01 mL, 8.02 mmol).
2,4-Dichloro-phenyl-biguanide (3e)
Compound 3e was obtained according to the general procedure, as a white powder from dicyandiamide 2 (311.38 mg, 3.70 mmol), 2,4-dichloroaniline 1e (600 mg, 3.70 mmol), and TMSCl (0.51 mL, 4.07 mmol).
3,4-Dichloro-phenyl-biguanide (3f)
Compound 3f was obtained according to the general procedure, as a beige powder from dicyandiamide 2 (518.77 mg, 6.17 mmol), 3,4-dichloroaniline 1f (1 g, 6.17 mmol) and TMSCl (0.86 mL, 6.79 mmol).
General procedure for the synthesis of 2,4-diamino-1,3,5-triazines
To a solution of corresponding biguanide, 3 (1 eq) in 15 ml of anhydrous methanol (MeOH) cooled at 0°C under nitrogen, were added sodium methanolate or sodium ethanolate (10 to 15 eq). To the cooled mixture, the corresponding ester derivative 4 (1.3 to 1.5 eq) was added. The reaction mixture was brought to room temperature and stirring was maintained for 30 min. Heating was conducted at reflux for 3h to 8h, depending on the nature of biguanide derivative 3. After refluxing, the mixture was cooled in ice crushed and the precipitate formed was filtered. The product was purified either by extraction or washing in organic solvent recrystallization or column chromatography. After purification, the 2,4-diamino-1,3,5-triazine derivatives (5a-k) were obtained as colored powders with yields from 16% to 86%.
6-(4-chlorophenyl)-N2-(4-(methylthio)phenyl)-1,3,5-triazine-2,4-diamine (5a)
Compound 5a was synthesized according to the general procedure from 4-methylthio-phenyl-biguanide hydrochloride 3a (1.05 g, 4.70 mmol), sodium methanolate (3 mL, 53.3 mmol), and 4-chlorobenzoate 4a (0.87 mL, 5.56 mmol). The precipitate formed was washed with dichloromethane (DCM), dried, and obtained as a beige powder with a yield of 16%. Mp 168 °C. 1H NMR (400 MHz, DMSO-d6), δ: 2.45 (s, 3H, SCH3), 7.24 (d, J = 8.3 Hz, 4H, H-2’’, H-6’’, NH2), 7.58 (d, J = 8.4 Hz, 2H, H-3’’, H-5’’), 7.79 (d, J = 8.1 Hz, 2H, H-3’, H-5’), 8.30 (d, J = 8.3 Hz, 2H, H-2’, H-6’), 9.60 (s, 1H, NH). 13C NMR (101 MHz, DMSO):15.89 (SCH3), 120.69 (C-2’’, C-6’’), 127.29 (C-2’, C-6’), 128.40 (C-3’, C-5’), 129.49 (C-4’’), 130.33 (C-3’’, C-5’’), 135.61 (C-1’), 136.13 (C-4’), 137.49 (C-1’’), 164.45 (C-4), 167.08 (C-6), 169.20 (C-2). IR, n(cm-1): 3317.56 (N-H), 1361.74 (CH3), 1658.78 (C=Car), 802.39 (C-Har). UPLC ESI/MS, [M+H]+: 344.8.
6-(4-chlorophenyl)-N2-(2-morpholinoethyl)-1,3,5-triazine-2,4-diamine (5b)
Compound 5b was synthesized according to the general procedure from 4-ethylmorpholinyl-biguanide hydrochloride 3b (1 g, 3.99 mmol), sodium methanolate (3 mL, 53.3 mmol) and 4-chlorobenzoate 4a (0.75 mL, 4.79 mmol). The reaction mixture was cooled, filtered, and evaporated under vacuum. The residue was put in water and extracted several times with diethyl ether. The organic phase was dried and evaporated to yield the expected product as a white powder with a yield of 34%. Mp 187 °C. 1H NMR (400 MHz, DMSO-d6), δ: 2.44 (m, 6H, H-2’, H-3’’’, H-5’’’), 3.36 - 3.50 (m, 2H, H-1’), 3.51 - 3.60 (m, 4H, H-2’’’, H-6’’’), 6.80 (br s, 1H, NH), 6.93 (s, 2H, NH2), 7.54 (m, 2H, H-3’’, H-5’’), 8.25 (m, 2H, H-2’’, H-6’’). 13C NMR (101 MHz, DMSO-d6), δ: 53.29 (C-1’), 57.41 (C-3’’’, C-5’’’, C-2’), 66.17 (C-2’’, C-6’’), 128.23 (C-2’’, C-6’’), 129.34 (C-3’’, C-5’’), 135.75 (C-1’’), 135.88 (C-4’’), 166.00 (C-6), 167.23 (C-4), 168.55 (C-2). IR, n(cm-1): 3267.41 (N-H), 2976.16 (C=CH2), 1604.77 (C=Car), 813.96 (C-Har). UPLC ESI/MS, [M+H]+: 335.8.
4-((4-amino-6-(4-chlorophenyl)-1,3,5-triazin-2-yl)amino)benzonitrile (5c)
Compound 5c was synthesized according to the general procedure from 4-cyanophenyl-biguanide hydrochloride 3c (1.05 g, 5.2 mmol), sodium methanolate (3 mL, 53.3 mmol), and 4-chlorobenzoate 4a (0.84 mL, 5.37 mmol). The precipitate formed was washed with water, and methanol and recrystallized from MeOH/H2O mixture (50/50). After filtration, the precipitate was dried and obtained as a white powder with a yield of 30%. Mp 298 °C. 1H NMR (400 MHz, DMSO-d6), δ : 7.42 (br s, 2H, NH2), 7.54 - 7.66 (m, 2H, H-2’’, H-6’’), 7.75 (d, J = 8.8 Hz, 2H, H-3’’, H-5’’), 8.06 (d, J = 8.8 Hz, 2H, H-3’, H-5’), 8.24 - 8.37 (m, 2H, H-2’, H-6’), 10.11 (s, 1H, NH), 13C NMR (101 MHz, DMSO-d6), δ : 103.24 (C-4’’), 119.41 (CN), 119.48 (C-2’’, C-6’’), 128.49 (C-2’, C-6’), 129.57 (C-3’, C-5’), 132.84 (C-3’’, C-5’’), 135.29 (C-1’), 136.39 (C-4’), 144.4 (C-1’’), 164.51 (C-6), 167.06 (C-4), 169.54 (C-2). IR, n(cm-1): 3317.56 (N-H), 1662.64 (C=Car), 802.39 (C-Har). UPLC ESI/MS, [M+H]+: 323.8.
N2-(benzo[d][1,3]dioxol-5-yl)-6-(4-chlorophenyl)-1,3,5-triazine-2,4-diamine (5d)
Compound 5d was synthesized according to the general procedure from benzo[d][1,3]dioxol-5-biguanide hydrochloride 3d (800 mg, 3.62 mmol), sodium methanolate (3 mL, 53.3 mmol) and 4-chlorobenzoate 4a (85 mL, 5.43 mmol). The precipitate formed was successively washed with methanol, water, and diethyl ether and dried. It was obtained as a green powder with a yield of 52%. Mp 206 °C. 1H NMR (400 MHz, DMSO-d6), δ: 5.98 (s, 2H, OCH2O), 6.85 (d, J = 8.4 Hz, 1H, H-5’), 7.14 (m, 3H, NH2, H-2’), 7.58 (m, 3H, H-3’’, H-5’’, H-6’), 8.28 (d, J = 8.4 Hz, 2H, H-2’’, H-6’’), 9.48 (s, 1H, NH). 13C NMR (101 MHz, DMSO-d6), δ: 100.75 (OCH2O), 102.48 (C-2’), 107.77 (C-5’), 112.80 (C-6’), 128.38 (C-2’’, C-6’’), 129.42 (C-3’’, C-5’’), 134.13 (C-1’’), 135.68 (C-4’’), 136.07 (C-1’), 142.21 (C-4’), 146.91 (C-3’), 164.38 (C-6), 167.05 (C-4), 169.09 (C-2). IR, n(cm-1): 3325.28 (N-H), 1658 (C=Car), 1473.62 (C=CH2), 808.17 (C-Har). UPLC ESI/MS, [M+H]+: 342.8.
6-(4-chlorophenyl)-N2-(2,4-dichlorophenyl)-2,4-diamino-1,3,5-triazine (5e)
Compound 5e was synthesized according to the general procedure from 2,4-dichloro-phenyl-biguanide hydrochloride 3e (450 mg, 1.83 mmol), sodium methanolate (3 mL, 53.3 mmol) and 4-chlorobenzoate 4a (0.43 mL, 2.74 mmol). The precipitate formed was successively washed with methanol, water, and cyclohexane and dried. It was obtained as a white powder with a yield of 40%. Mp. 239 °C. 1H NMR (400 MHz, DMSO-d6), δ: 7.18 (br s, 2H, NH2), 7.44 (dd, J = 8.7, 2.4 Hz, 1H, H-6’’), 7.56 (d, J = 8.6 Hz, 2H, H-3’, H-5’), 7.68 (d, J = 2.4 Hz, 1H, H-5’’), 7.78 (d, J = 8.7 Hz, 1H, H-3’’), 8.23 (d, J = 8.5 Hz, 2H, H-2’, H-6’), 8.97 (s, 1H, NH). 13C NMR (101 MHz, DMSO-d6), δ: 127.37 (C-6’’), 128.48 (C-4’’), 128.58 (C-2’’), 128.79 (C-5’’), 129.16 (C-2’, C-6’), 129.48 (C-3’, C-5’), 129.59 (C-3’’), 134.95 (C-1’), 135.36 (C-4’), 136.21 (C-1’’), 165.11(C-6), 167.21 (C-4), 169.33 (C-2). IR, n(cm-1): 3342.64 (N-H), 1591.27 (C=Car, 804.32 (C-Har). UPLC ESI/MS, [M+H]+: 367.6.
6-(4-chlorophenyl)-N2-(3,4-dichlorophenyl)-2,4-diamino-1,3,5-triazine (5f)
Compound 5f was synthesized according to the general procedure from 3,4-dichloro-phenyl-biguanide hydrochloride 3f (500 mg, 2.03 mmol), sodium methanolate (3 mL, 53.3 mmol) and 4-chlorobenzoate 4a (0.48 mL, 3.045 mmol). The precipitate formed was washed with methanol, water, and cyclohexane and dried. It was obtained as a white powder with a yield of 45%. Mp. 252°C 1H NMR (400 MHz, DMSO-d6), δ: 7.37 (sl, 2H, NH2), 7.54 (d, J = 8.8 Hz, 1H, H-2’’), 7.60 (d, J = 8.5 Hz, 2H, H-3’, H-5’), 7.80 (dd, J = 8.8, 2.3 Hz, 1H, H-2’’), 8.22 (d, J = 1.8 Hz, 1H, H-5’’), 8.29 (d, J = 8.5 Hz, 2H, H-2’, H-6’), 9.90 (s, 1H, NH). 13C NMR (101 MHz, DMSO-d6), δ : 119.67 (C-6”), 120.78 (C-2’’), 123.23 (C-4’’), 128.47 (C-2’, C-6’), 129.48 (C-3’, C-5’), 130.18 (C-5’’), 130.72 (C-3’’), 135.37 (C-1’), 136.33 (C-4’), 140. 14 (C-1’’), 164.39 (C-6), 167.02 (C-4), 169.39 (C-2). IR, n(cm-1): 3342.64 (N-H), 1591.27 (C= Car), 804.32 (C-Har). UPLC ESI/MS, [M+H]+: 367.6.
N-2-(4-(methylthio)phenyl)-6-(pyridin-2-yl)-1,3,5-triazine-2,4-diamine (5g)
Compound 5g was synthesized according to the general procedure from 4-thiomethyl-phenyl-biguanide hydrochloride 3g (525 mg, 2.35 mmol), sodium ethanolate (3 mL, 38.26 mmol), and ethyl-2-picolinate 4b (0.41 mL, 3.05 mmol). The reaction mixture was evaporated and the solid obtained was recrystallized from a MeOH/H2O mixture (1/2). It was obtained as a beige powder with a yield of 23%. Mp 172 °C. 1H NMR (400 MHz, DMSO-d6), δ : 2.45 (s, 3H, CH3), 7.26 (m, 4H, H-2’, H-6’, NH2), 7.54 (m, 1H, H-4’’), 7.83 (d, J = 8.7 Hz, 2H, H-3’, H-5’), 7.96 (td, J = 7.7, 1.7 Hz, 1H, H-5’’), 8.26 (d, J = 7.9 Hz, 1H, H-6’’), 8.71 (d, J = 4.6 Hz, 1H, H-3’’), 9.78 (s, 1H, NH). 13C NMR (101 MHz, DMSO-d6), δ : 15.91 (CH3), 120.50 (C-2’, C-6’), 123.23 (C-4’’), 125.41 (C-6’’), 127.31 (C-4’), 130.17 (C-3’, C-5’), 136.70 (C-1’), 137.63 (C-5’’), 149.23 (C-3’’), 154.34 (C-1’’), 164.67 (C-6), 167.33 (C-2), 170.17 (C-4). IR, n(cm-1): 3180.62 (N-H), 1606.70 (C=Car), 1369.46 (CH3), 825.33 (C-Har). UPLC ESI/MS, [M+H]+: 311.4
N2-(2,4-dichlorophenyl)-6-(pyridin-2-yl)-2,4-diamino-1,3,5-triazine (5h)
Compound 5h was synthesized according to the general procedure from 2,4-dichloro-phenyl-biguanide hydrochloride 3h (400 mg, 1.625 mmol), 3 mL of sodium ethanolate (38.26 mmol) and ethyl-2-picolinate 4b (0.44 mL, 3.25 mmol). The reaction mixture was evaporated. Then, the solid formed was purified on column chromatography with a DCM/MeOH mixture (9/1). After evaporation under vacuum, the dry residue was washed with DCM and was obtained as a pink powder with a yield of 45%. Mp 230 °C. 1H NMR (400 MHz, DMSO-d6), δ: 7.02-8.04 (m, 7H, H-3’, H-5’, H-6’, H-4’’, H-5’’, NH2), 8.20 (d, J = 7.7 Hz, 1H, H-6’’), 8.69 (d, J = 3.8 Hz, 1H, H-3’’), 9.07 (s, 1H, NH). 13C NMR (101 MHz, DMSO-d6), δ: 123.31 (C-6’), 125.47 (C-4’’), 127.39 (C-6’’), 128.50 (C-4’), 128.80 (C-5’), 129.23 (C-2’), 135.00 (C-3’), 136.72 (C-1’), 149.25 (C-5’’), 149.25 (C-3’’), 154.17 (C-1’’), 165.38 (C-6), 167.50 (C-2), 170.40 (C-4). IR, n (cm-1): 3113.11 (N-H), 1606,70 (C=Car), 812,03 (C-Har). UPLC ESI/MS, [M+H]+: 334.2.
N2-(3,4-dichlorophenyl)-6-(pyridin-2-yl)-2,4-diamino-1,3,5-triazine (5i)
Compound 5i was synthesized according to the general procedure from 3,4-dichloro-phenyl-biguanide hydrochloride 4i (510 mg, 2.07 mmol), 3 mL of sodium ethanolate (38.26 mmol), and ethyl-2-picolinate 4b (0.56 mL, 4.14 mmol). The precipitate formed was successively washed with methanol, water, and diethyl ether and dried. It was obtained as a pink powder with a yield of 86%. Mp 243 °C. 1H NMR (400 MHz, DMSO-d6), δ: 7.22-7.67 (m, 4H, H-2’, H-5’, NH2), 7.84 (d, J = 8.5 Hz, 1H, H-4’’), 7.98 (t, J = 7.2 Hz, 1H, H-6’), 8.29 (m, 2H, H-5’’, H-6’’), 8.72 (d, J = 3.3 Hz, 1H, H-3’’), 10.11 (s, 1H, NH). 13C NMR (101 MHz, DMSO-d6), δ: 119.55 (C-6’), 120.66 (C-2’), 123.18 (C-4’), 123.27 (C-4’’), 125.67 (C-6’’), 130.16 (C-5’), 130.75 (C-3’), 136.88 (C-5’’), 140.23 (C-1’), 149.36 (C-3’’), 153.94 (C-1’’), 164.63 (C-6), 167.19 (C-2), 170.20 (C-4). UPLC ESI/MS, [M+H]+: 334.03. IR, n(cm-1): 3147.83 (N-H), 1598.99 (C=Car), 790.81 (C-Har). UPLC ESI/MS, [M+H]+: 334.2.
N2-(benzo[d][1,3]dioxol-5-yl)-6-(pyridin-4-yl)-2,4-diamino-1,3,5-triazine (5j)
Compound 5j was synthesized according to the general procedure from benzo[d][1,3]dioxol-5-biguanide hydrochloride 4j (650 mg, 2.94 mmol), sodium ethanolate (3 mL, 38.26 mmol) and ethyl isonicotinate 4c (0.57 mL, 3.822 mmol). The precipitate formed was successively washed with methanol, water and DCM then dried. It was obtained as a grey powder with a yield of 24%. Mp 218 °C. 1H NMR (400 MHz, DMSO-d6), δ: 5.99 (s, 2H, CH2), 6.86 (d, J = 8.4 Hz, 1H, Hb), 7.15 (dd, J = 8.4, 1.8 Hz, 1H, Ha), 7.31 (s, 2H, NH2), 7.58 (s, 1H, He), 8.11 (d, J = 5.5 Hz, 2H, Hg), 8.76 (d, J = 5.7 Hz, 2H, Hh), 9.60 (s, 1H, NH). 13C NMR (101 MHz, DMSO-d6), δ: 100.79 (OCH2O), 102.59 (C-2’), 107.79 (C-5’), 112.97 (C-6’), 121.46 (C-2’’, C-6’’), 133.93 (C-1’), 142.37 (C-1’’), 144.21 (C-3’’), 146.93 (C-5’’), 150.18 (C-6), 164.42 (C-4), 167.13 (C6), 168.64 (C4). IR, n(cm-1): 3180.62 (N-H), 1591.27 (C=Car), 1487.12 (C=CH2), 802.39 (C- Har). UPLC ESI/MS, [M+H]+: 309.10.
N2-(3,4-dichlorophenyl)-6-(pyridin-4-yl)-1,3,5-triazine-2,4-diamine (5k)
Compound 5k was synthesized according to the general procedure from 1-(3,4-dichlorophenyl)biguanide hydrochloride 3k (500 mg, 2.03 mmol), sodium ethanolate (3 mL, 38.26 mmol), and ethyl isonicotinate 4c (0.39 mL, 2.64 mmol). The precipitate formed was successively washed with methanol, water, and diethyl ether and then dried. It was obtained as a pink powder with a yield of 36%. Mp 276 °C. 1H NMR (400 MHz, DMSO-d6), δ: 7.55 (m, 3H, H-2’, NH2), 7.81 (dd, J = 8.9, 2.4 Hz, 1H, H-5’), 8.12 (d, J = 5.9 Hz, 2H, H-2’’, H-6’’), 8.22 (d, J = 1.7 Hz, 1H, H-6’), 8.77 (d, J = 5.8 Hz, 2H, H-3’’, H-5’’), 10.01 (s, 1H, NH). 13C NMR (101 MHz, DMSO-d6), δ: 119.81 (C-6’), 120.92 (C-2’), 121.49 (C-6’’, C-2’’), 123.47 (C-4’), 130.23 (C-5’), 130.75 (C-3’), 139.98 (C-1’), 143.91 (C-1’’), 150.27 (C-3’’, C-5’’), 164.46 (C-6), 167.11 (C-4), 168.96 (C-2). IR, n(cm-1): 3307.92 (N-H), 1600.32 (C=Car), 804.32 (C-Har). UPLC ESI/MS, [M+H]+: 334.03.
RESULTS AND DISCUSSION
Chemistry
In this work, novel triazines were synthesized in two steps. The first step consisted of adding primary amines 1 with dicyandiamide 2 in the presence of chlorotrimethylsilane in acetonitrile, under microwave irradiation for 10 min at 130 °C (18 bar, 60 W). The intermediates resulting from this first step were biguanides 3a-f (Figure 1).
|
|
|
Figure 1. Synthesis of biguanides |
At the end of the reaction, the biguanide formed was cooled in ice crushed, filtered, and washed with acetonitrile and isopropanol. The resulting biguanide in powdery form was dried and used without further purification for the next step [18]. The yields of synthesized biguanides ranged from 61-95% (Table 1).
|
Molecules |
Structures |
Yields (%) |
|
3a |
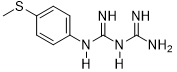 |
61 |
|
3b |
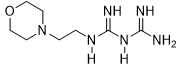 |
95 |
|
3c |
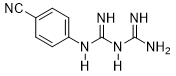 |
92 |
|
3d |
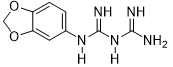 |
76 |
|
3e |
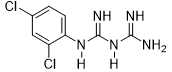 |
70 |
|
3f |
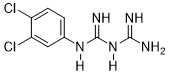 |
50 |
|
|
Then, biguanides previously obtained were condensed with various esters of interest according to the reaction scheme (Figure 2).
|
|
|
Figure 2. Synthesis of 6-aryl-2,4-diamino-1,3,5-triazines |
The reaction was carried out under an inert atmosphere, at the reflux of ethanol or methanol according to the chosen base (EtONa or MeONa [19]). The reaction was followed by TLC and UPLC-MS. After completion of the reaction, synthesized triazines 5a-k precipitated. The precipitate obtained was recovered by filtration and washed successively with methanol and dichloromethane. Depending on its solubility, it was rinsed with ethyl acetate, diethyl ether, or cyclohexane. If necessary, it was either recrystallized in a methanol/water mixture 1:1 or 1:2 or purified on a silica gel column with a convenient solvent. The yields of 6-aryl-2,4-diamino-1,3,5-triazines (5a-k) ranged from 16-86% (Table 2).
|
Molecules |
R1 |
R2 |
Yields (%) |
|
5a |
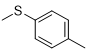 |
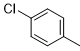 |
16 |
|
5b |
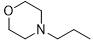 |
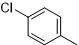 |
34 |
|
5c |
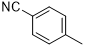 |
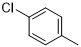 |
30 |
|
5d |
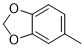 |
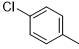 |
52 |
|
5e |
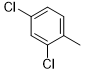 |
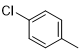 |
40 |
|
5f |
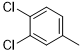 |
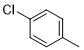 |
45 |
|
5g |
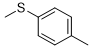 |
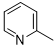 |
23 |
|
5h |
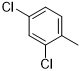 |
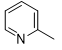 |
45 |
|
5i |
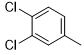 |
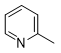 |
86 |
|
5j |
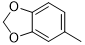 |
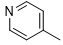 |
24 |
|
5k |
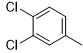 |
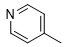 |
36 |
Biological section
Table 3. Experimental conditions used for protein kinase assays.
|
(Family) |
Enzyme Description |
Substrate* (Working concentration) |
Buffer used** |
|
GSK3α (CMGC) |
Human, recombinant, expressed by baculovirus in Sf9 insect cells |
GS-1 peptide: YRRAAVPPSPSLSRHSSPHQSpEDEEE *** (20 µM) |
A |
|
GSK3β (CMGC) |
Human, recombinant, expressed by baculovirus in Sf9 insect cells |
GS-1 peptide: YRRAAVPPSPSLSRHSSPHQSpEDEEE *** (20 µM) |
A |
|
CDK5/p25 (CMGC) |
Human, recombinant, expressed in bacteria |
Histone H1 (37.2 µM) |
A |
|
EGFR (TK) |
Human, recombinant, expressed by baculovirus in Sf9 insect cells |
Poly(L-glutamic acid – L-tyrosine) sodium salt (0.17 µg/µL) |
A |
|
(TK) |
Human, recombinant, expressed by baculovirus in Sf9 insect cells |
Poly(L-glutamic acid – L-tyrosine) sodium salt (0.17 µg/µL) |
A |
|
EphB1 (TK) |
Human, recombinant, expressed by baculovirus in Sf9 insect cells |
Poly(L-glutamic acid – L-tyrosine) sodium salt (0.17 µg/µL) |
A |
|
JAK3 (TK) |
Human, recombinant, expressed by baculovirus in Sf9 insect cells |
Peptide: GGEEEEYFELVKKKK (94 µM) |
A |
|
ABL1 (TK) |
Human, recombinant, expressed by baculovirus in Sf9 insect cells |
Peptide: EAIYAAPFAKKK (127 µM) |
A |
|
DYRK1A (CMGC) |
Human recombinant, expressed by baculovirus in Sf9 insect cells |
Peptide: KKISGRLSPIMTEQ (10.7 µM) |
A |
|
CLK1 (CMGC) |
Human, recombinant, expressed by baculovirus in Sf9 insect cells |
Peptide: GRSRSRSRSRSR (57.3 µM) |
A |
|
CK1ε (CK1) |
Human, recombinant, expressed by baculovirus in Sf9 insect cells |
Peptide: RRKHAAIGSpAYSITA *** (170 µM) |
A |
|
PIM1 (CAMK) |
Human proto-oncogene, recombinant, expressed in bacteria |
PimTide: ARKRRRHPSGPPTA (630 µM) |
A |
* Peptide substrates were obtained from ProteoGenix (Schiltigheim, France) or Sigma for Histone H1, Poly (L-glutamic acid – L-tyrosine) sodium salt
** Composition of the buffers: Buffer A: 10 mM MgCl2, 1 mM EGTA, 1 mM DTT, 25 mM Tris-HCl pH 7.5, 50 µg/mL heparin
*** “Sp’’ stands for phosphorylated serine
Primary screening and IC50 determination
In this study, synthesized triazines were tested on 12 protein kinases (GSK3-a/b, CDK5/p25, EGFR, VEGFR2, EphB1, JAK3, ABL1, DYRK1A, CLK1, CK1ε, and PIM1) (Table 3) to evaluate their potential enzymatic activity. Kinase activities were measured using the ADP-GloTM bioluminescent kinase assay kit (Promega, Madison, WI) according to the recommendations of the manufacturer, described according to the protocol of Zegzouti et al. [20]. These enzymatic activities were detected in the presence of 10 µM ATP. Reactions were carried out in the presence of 10 µM of ATP, in a final volume of 6 µl for 30 min at 30 °C in ADP-Glo buffer (25 mM Tris-HCl pH 7.5, 10 mM MgCl2, 1 mM EGTA, 1 mM DTT, 50 µg/mL heparin and 0.1 mg/ml of BSA). After that, 6 µl of ADP-GloTM Kinase Reagent was added to stop the kinase reaction. After an incubation time of 50 min at room temperature, 12 µl of ADP-GloTM Kinase Detection Reagent was added for one hour at room temperature. The transmitted signal was measured using the Envision (PerkinElmer, Waltham, MA) microplate luminometer and expressed in Relative Light Unit (RLU). Kinase activities were expressed in % of maximal activity, i.e. measured in the absence of an inhibitor. In primary screening, chemical compounds, dissolved in DMSO, are tested at 10 µM and 1 µM final in the kinase assay. To determine the half-maximal inhibitory concentration (IC50), the assays were performed in duplicate in the absence or presence of increasing doses of the tested compounds. Positive controls (for total activity) and negative controls (for background noise) were performed with appropriate dilutions of dimethylsulfoxide (DMSO). Note that a value ≥ 100 indicates that the compound tested can not inhibit the enzymatic activity of the kinase at the concentration tested (Table 4).
Table 4. Primary screening of triazines.
|
Compound |
Concentration |
GSK3α |
GSK3β |
CDK5/p25 |
EGFR |
VEGFR2 |
EphB1 |
JAK3 |
ABL1 |
DYRK1A |
CLK1 |
CK1ε |
PIM1 |
|
5a |
10 µM |
90 |
86 |
89 |
≥ 100 |
≥ 100 |
42 |
55 |
≥ 100 |
85 |
71 |
55 |
43 |
|
1 µM |
≥ 100 |
87 |
≥ 100 |
89 |
≥ 100 |
70 |
71 |
≥ 100 |
102 |
99 |
≥ 100 |
≥ 100 |
|
|
5b |
10 µM |
≥ 100 |
≥ 100 |
≥ 100 |
≥ 100 |
78 |
≥ 100 |
≥ 100 |
≥ 100 |
92 |
65 |
≥ 100 |
27 |
|
1 µM |
≥ 100 |
≥ 100 |
89 |
≥ 100 |
85 |
90 |
≥ 100 |
≥ 100 |
≥ 100 |
100 |
≥ 100 |
55 |
|
|
5c |
10 µM |
100 |
≥ 100 |
67 |
79 |
96 |
48 |
18 |
≥ 100 |
86 |
67 |
97 |
74 |
|
1 µM |
97 |
97 |
≥ 100 |
100 |
100 |
91 |
81 |
≥ 100 |
92 |
99 |
≥ 100 |
97 |
|
|
5d |
10 µM |
68 |
71 |
67 |
70 |
66 |
44 |
59 |
87 |
90 |
71 |
44 |
46 |
|
1 µM |
83 |
85 |
87 |
78 |
70 |
74 |
59 |
86 |
≥ 100 |
≥ 100 |
93 |
≥ 100 |
|
|
5e |
10 µM |
94 |
89 |
96 |
≥ 100 |
≥ 100 |
95 |
77 |
86 |
≥ 100 |
≥ 100 |
87 |
62 |
|
1 µM |
100 |
82 |
99 |
≥ 100 |
≥ 100 |
73 |
86 |
74 |
98 |
≥ 100 |
≥ 100 |
≥ 100 |
|
|
5f |
10 µM |
≥ 100 |
91 |
86 |
97 |
≥ 100 |
48 |
74 |
57 |
≥ 100 |
≥ 100 |
90 |
82 |
|
1 µM |
≥ 100 |
91 |
90 |
97 |
≥ 100 |
61 |
51 |
50 |
96 |
≥ 100 |
100 |
≥ 100 |
|
|
5g |
10 µM |
98 |
80 |
≥ 100 |
90 |
≥ 100 |
29 |
76 |
77 |
91 |
≥ 100 |
78 |
77 |
|
1 µM |
≥ 100 |
81 |
98 |
99 |
≥ 100 |
66 |
78 |
73 |
92 |
≥ 100 |
95 |
≥ 100 |
|
|
5h |
10 µM |
79 |
78 |
83 |
81 |
72 |
53 |
70 |
60 |
≥ 100 |
98 |
97 |
78 |
|
1 µM |
78 |
96 |
≥ 100 |
70 |
62 |
56 |
73 |
53 |
≥ 100 |
≥ 100 |
110 |
99 |
|
|
5i |
10 µM |
76 |
≥ 100 |
≥ 100 |
69 |
50 |
55 |
83 |
56 |
≥ 100 |
97 |
93 |
86 |
|
1 µM |
66 |
60 |
≥ 100 |
70 |
51 |
70 |
45 |
54 |
≥ 100 |
≥ 100 |
≥ 100 |
≥ 100 |
|
|
5j |
10 µM |
68 |
85 |
68 |
74 |
49 |
73 |
57 |
58 |
72 |
51 |
71 |
≥ 100 |
|
1 µM |
96 |
≥ 100 |
≥ 100 |
93 |
80 |
82 |
76 |
87 |
96 |
89 |
≥ 100 |
≥ 100 |
|
|
5k |
10 µM |
96 |
97 |
83 |
≥ 100 |
91 |
58 |
≥ 100 |
124 |
49 |
50 |
57 |
77 |
|
1 µM |
≥ 100 |
93 |
≥ 100 |
≥ 100 |
104 |
43 |
≥ 100 |
99 |
62 |
89 |
90 |
89 |
Primary screening results highlighted seven compounds 5a, 5b, 5c, 5d, 5g, 5i and 5k, which inhibited kinases EphB1, JAK3, CK1Ɛ, and PIM1. Enzymatic activity percentages of these compounds, at 10 and 1 μM concentrations, were noticed in Table 4. Dose-response studies were subsequently undertaken with IC50 determination. IC50 of active molecules which obtained the best results were determined in Table 5.
Table 5. IC50 determination at 10 µM for active triazines
|
Compounds |
IC50 (µg/mL) |
||
|
EphB1 |
PIM1 |
JAK3 |
|
|
5a |
3.07 |
- |
- |
|
5b |
- |
1.18 |
- |
|
5c |
- |
- |
1.56 |
|
5d |
- |
1.76 |
- |
|
5g |
15.43 |
- |
- |
From these different results, we were able to identify two chemical cores favorable to the inhibition of enzymatic activity. These are 6-chlorophenyl-1,3,5-triazine and 6-(pyridin-2-yl)-1,3,5-triazine which carry an aminophenyl group in position 2. The latter must be monosubstituted in the para position of amine function by a thiomethyl or nitrile group (compounds 5a, 5c, and 5g). On the one hand, polysubstitution in the ortho and para position or the meta and para position by chlorine atoms led to inactivity on kinases. On the other hand, introduction into position 2, on 6-chlorophenyl-2,4-diamino-1,3,5-triazine of ethylamino-morpholinyl substituent (compound 5b) or 5-aminobenzodioxole (compound 5d) caused inhibition of protein kinases. Moreover, the most active molecule was compound 5b which inhibited PIM1 kinase (IC50 = 1.18 µg/mL), Table 5. However, introduction at position 6 of the pyridine-4-yl group on 1,3,5-triazine moiety resulted in a lack of enzymatic activity, even though this structural variation was coupled with the presence, in position 2, of the group previously favorable to inhibition such as 5-aminobenzodioxole (5j).
CONCLUSION
Here, we have reported the synthesis in two steps and the biological evaluation of a series of new triazines 5a-k. We successfully developed a library of 11 new compounds with overall yields ranging from 16-86%. For the first step, we used microwave irradiation for the synthesis of biguanides 3a-e. This approach allowed structural diversity of intermediates biguanides 3a-e which led to final triazines. Enzymatic inhibition was measured on all final compounds. Results on protein kinase assays showed that the most active molecule was compound 5b which inhibited PIM1 kinase with the value of IC50 = 1.18 µg/mL. This result is the starting point of a larger research program within our group that can investigate the introduction of substituted groups on triazine’s terminal amino group. Therefore, the perspectives identified will concern the search for new pharmaco-modulations and structural optimization favorable to the increase of protein kinases inhibitory activity involved in immune pathophysiology, inflammatory or cancerous diseases, to generate lead compounds, and be able to conduct studies on relevant in vivo models.
ACKNOWLEDGMENTS: We would like to thank Cancéropôle Grand Ouest for its support for this work as well as the different teams of the network with whom we have had constructive exchanges.
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: This work was financially supported by the Cooperation and Cultural Action Service of the French Embassy in Côte d'Ivoire (SCAC).
ETHICS STATEMENT: None