Chronic conditions known as Inflammatory Bowel Disease (IBD), including Crohn's Disease (CD) and Ulcerative Colitis (UC), have a significant impact on the quality of life experienced by patients. Innovative research is revealing complex molecular interactions between medicinal plants and diseases, advancing plant-based therapies. Usually, taking medicine and changing one's lifestyle are the main ways to manage the illness. Recent studies, however, suggest that medicinal plants may offer further, helpful choices for treating the illness. This review article examines around 99 articles from sources such as Scopus, Web of Science, Google Scholar, PubMed, Researchgate, DOAJ, SCIELO, and NOPR. The review methodically investigates the therapeutic potential of different medicinal plants in the management of IBD, including Crohn's disease and ulcerative Colitis. The review provides insights into the underlying mechanisms, pharmacological properties, and effectiveness of these plants. The findings present a promising opportunity for further research and development of plant-based therapies to improve IBD management and improve patient outcomes.
INTRODUCTION
The two most widespread forms of chronic conditions causing inflammation in the gastrointestinal (GI) tract are Crohn's Disease (CD) and Ulcerative Colitis (UC). Although the exact cause remains unclear, experts believe it was a fusion of genetic, environmental, and immunological factors that led to an outbreak of abnormal immune response in the GI tract lining. CD disease can impact any section from mouth to anus, but it typically affects the ileum and colon most frequently. The condition is marked by patchy inflammation, and it can cause thickening of the bowel wall and deep ulcers. On the other hand, UC exclusively impacts the colon and is distinguished by persistent inflammation of the innermost layer, resulting in the formation of ulcers followed by bloody diarrhea. Inflammatory Bowel Disease (IBD) symptoms can differ, with some individuals experiencing abdominal pain, fatigue, weight loss, and malnutrition. Potential complications associated with IBD encompass bowel obstructions, fistulas, and a heightened risk of colorectal cancer.
Managing IBD usually requires a combination of medications, lifestyle changes, and occasionally surgery. Although no cure exists for IBD, treatment aims to control symptoms, sustain remission, and enhance the patient’s quality of life.
IBD's exact cause is unknown, but several risk factors may increase the likelihood of developing the condition. Genetic factors include numerous genes associated with IBD susceptibility, while environmental factors encompass diet, smoking, use of NSAIDs, and urban living. An overactive immune response and an imbalanced gut microbiome also play roles in the development of IBD. However, having one or more risk factors does not guarantee IBD development, and many affected individuals have no known risk factors.
Indian traditional medicine is a holistic healthcare system focused on balancing the body, mind, and spirit. It uses personalized treatment plans, natural remedies, and preventive measures to promote overall wellness. Key advantages of Indian traditional medicine include its general approach, tailored treatments, natural remedies, emphasis on prevention, and recognition of the mind-body connection. Indian traditional medicine and Western medicine have their unique advantages and applications in treating IBD. Integrating the best practices from both systems can provide a comprehensive approach to healthcare, offering patients the most effective and personalized treatments.
The significant healing benefits of Indian medicinal plants for IBD have garnered growing interest among researchers. The primary focus of utilizing Indian medicinal plants for IBD therapy is to enhance the effectiveness of treatment by reducing inflammation, promoting gut health, and alleviating symptoms by minimizing potential side effects. These plants are believed to work synergistically with conventional therapies for patients with a more holistic approach to managing their condition. This article highlights the advancements in basic research made by Indian medicinal plants for IBD treatment in recent years.
Common symptoms of IBD
CD and UC, the two most common forms of IBD, share similarities in their impact on the gastrointestinal system and chronic inflammation, yet they exhibit distinct manifestations. CD can affect any part of the GI system, but it most commonly involves the terminal ileum and the ascending colon. However, UC is still only seen in the large intestine (colon) and rectum. Inflammation caused by CD can spread throughout the entire gut wall, whereas UC normally affects only the mucosal layer of the colon. Inflammation in CD often occurs in discrete locations in the gastrointestinal tract, with healthy tissue intervening. In UC, the inflammation is persistent and spreads from the rectum to the rest of the colon. However, there is some overlap in the symptoms between the two diseases. It's crucial to remember that symptoms can be mild, moderate, or severe and that they can go into remission or flare up at any time.
UC specific symptoms
Abdominal pain and cramping are typical symptoms of both CD and UC, with pain varying in intensity and occurring persistently or intermittently. Diarrhea is a common feature in both conditions, characterized by frequent loose or watery stools. Rectal bleeding, although more prevalent in UC, can also occur in CD. The urgency to defecate is a frequent symptom, especially in UC, leading to a sudden and pressing need for a bowel movement. Unintentional weight loss may occur due to reduced appetite, malabsorption of nutrients, or inflammation. Fatigue is another common complaint, resulting from persistent inflammation, malnutrition, or anemia. Additionally, during active inflammation or flare-ups, individuals may experience low-grade fever as a symptom of CD or UC.
CD specific symptoms
In addition to the previously mentioned symptoms, CD can lead to mouth ulcers, which are painful sores that develop in the mouth. Furthermore, individuals with CD may experience anal fissures or fistulas, characterized by cracks in the skin around the anus or abnormal connections between the bowel and other structures. On the other hand, UC presents specific symptoms, such as tenesmus, which is a constant urge to have a bowel movement even when the bowel is empty, and increased mucus in the stool due to heightened production in the rectum and colon. Both CD and UC can also manifest extraintestinal symptoms, affecting other organs and systems in the body. These extraintestinal manifestations may include joint pain, skin rashes, eye inflammation, and liver problems.
MATERIALS AND METHODS
In this study, we used main sources such as Scopus, Web of Science, Google Scholar, PubMed, Research Gate, DOAJ, SCIELO, and NOPR. By searching keywords like "Inflammatory bowel diseases," "Crohn's disease," "Ulcerative colitis," and potential medicinal plant names, we collected 122 articles. We then organized them by language, topic, field, and publication year. Our review specifically targeted in vivo and in vitro studies published in English between January 2000 and March 2022. Peer-reviewed scientific literature is generally considered more reliable than other research types. Our comprehensive review aimed to explore and discuss the use of medicinal plants in treating inflammatory bowel diseases.
RESULTS AND DISCUSSION
Medicinal plants for inflammatory bowel disease
Aegle marmelos (L.) Correa
Originating from the Indian subcontinent, the Aegle marmelos (A. marmelos) plant is employed in Ayurvedic medicine as well as traditional folk medicine for the treatment of various ailments. Plant components are put to good use, from leaves and bark to roots and fruits to seeds. In animal models of IBD, a polyherbal Ayurvedic preparation containing Bilwa (A. marmelos), Dhanyak (Coriandrum sativum), Musta (Cyperus rotundus), and Vala (Vetiveria zinzanioids) has demonstrated considerable inhibitory efficacy. This efficacy is on par with that of the gold standard medication prednisolone. The results suggested that Cyperus rotundus was the principal active ingredient, with the other herbs perhaps relieving symptoms and fighting intestinal infections. Moreover, the composition of the formulation can potentially impact the generation or release of inflammatory mediators [1]. Wistar albino rats with acetic acid (AA) and indomethacin-induced UC and enterocolitis were given oral administrations of A. marmelos unripe fruit extract (AMFE). The treatment demonstrated a dose-dependent decrease in intestinal inflammation, preservation of mast cells, and enhancement of antioxidant activity, suggesting that AMFE's anti-inflammatory effect, possibly attributed to its phytochemical constituents like flavonoids, phenolic compounds, and steroids, holds promise for the potential protective treatment of IBD [2]. Another study involved administering daily doses of 100, 200, and 400 mg/kg of ethanolic extract obtained from the dried fruit pulp of A. marmelos (AME) to rats with AA-induced colitis for 14 days, revealing that the most pronounced protective effect was observed with a 200 mg/kg dosage of AME. The treatment with AME improved body weight and reduced colonic mucosa damage, inflammation, bloody or mucous diarrhea, and stool output in rats. AME also significantly improved antioxidant activity, decreased free radicals and myeloperoxidase activity, and showed substantial antibacterial activity [3]. When given orally to rats with TNBS-induced colitis, the 50% ethanol extract of AME reduced colonic damage, inflammation, diarrhea, free radicals, myeloperoxidase levels, and body weight while increasing antioxidants in the colon. The antibacterial activity of AME against intestinal pathogens suggests it may be useful in treating TNBS-induced experimental colitis. In terms of effectiveness, AME was on par with the well-known colitis-protective medication sulfasalazine [4]. HPLC analysis revealed the presence of umbelliferon and lupeol in AME. DSS-induced colitis in Swiss albino mice, treated with oral administration of AME at a dosage of 50 mg/kg resulted in a significant reduction of sickness symptoms and mRNA expressions of pro-inflammatory mediators IL-2, IL-6, and tumor necrosis factor. The potential of AME to lower NF-kB expression in the colon further aided histopathological improvements [5]. A. marmelos leaf extract can prevent AA-induced enterocolitis in rats by reducing oxidative stress and improving physiological parameters [6]. A. marmelos, Bombax malabericum, and Hollarrhena antidysentrica plant extracts were combined in varying proportions to create the formula. To determine the optimal dosage, an experimental Box-Behnken design was implemented. The CMDI and DAI values seen in the control batch indicated that the treatment regimen of 100 mg/kg A. marmelos, 300 mg/kg Bombax malabericum, and 200 mg/kg Holarrhena antidysentrica was successful in alleviating IBD symptoms [7].
Aloe vera (A. vera) gel contains over 200 active components that promote health, making it a rich natural source for human well-being. Imbalances between oxidants and antioxidants may be one of the reasons cause IBD, and antioxidants help combat free radicals and inflammation. A. vera gel's antioxidant properties derive from its GPx activity, SOD enzymes, and phenolic antioxidants. A dose-dependent radical quencher effect was witnessed in inflamed colorectal tissue and two cell-free in vitro systems. These cell-free methods evaluated superoxide and peroxyl radical scavenging. Furthermore, a 1:50 concentration of A. vera gel hindered prostaglandin E2 production in inflamed colorectal samples without affecting thromboxane B2 release [8]. A natural antioxidant preparation made from A. vera and ubiquinol can help reduce inflammation and free radical-induced stress in rats with DSS-caused colitis. The treatment proves effective solely when administered before the onset of colitis, rather than during or after [9]. By lowering inflammation, ulcer score, and tissue injury in rats with AA-triggered colitis, A. vera gel (50 and 300 mg/kg) demonstrated promising preventive and therapeutic effects compared to a water-based control. Additionally, sulfasalazine (100 mg/kg) and A. vera gel (50 and 300 mg/kg) pre-treatment showed similar therapeutic effects in decreasing inflammation, lesions, and fibrosis [10]. Histopathological and gene expression changes in the colon tissue samples of rats with AA-resulted in UC were analyzed to determine the therapeutic effects of A. vera gel. A. vera gel reduced colon tissue damage and apoptosis in rats by downregulating Bax mRNA and upregulating BCL-2. The expression of Bax mRNA is downregulated and the expression of Bcl-2 mRNA is upregulated, both of which are beneficial in the therapy of UC in rats treated with A. vera gel [11]. Rats with experimental colitis caused by TNBS may benefit from treatment with an A. vera extract extracted from ethanol. When compared to oral treatment, 400 mg/kg of A. vera extract administered rectally was more efficient in reducing serum concentrations of inflammatory agents and oxidative stress indicators, improving body weight and colon weight/length ratios [12]. The hypothesis suggests that A. vera gel has the potential to alleviate UC in male Wistar rats induced by AA. Various factors like clinical activity index, swelling, tissue sample alterations, DNA quantity in colon cells, and nitric oxide levels are crucial markers for disease progression. Notably, the highest dosage of A. vera gel (60 mg/kg body weight) exhibited greater effectiveness compared to dexamethasone [13]. Aloin A (A. vera), a major compound in A. vera, can boost the intestinal barrier role and be a future treatment for UC by preventing colitis symptoms and mitigating inflammation response, promoting colon cell proliferation, up-regulating expression of tight junction (TJ) proteins, and inhibiting the Notch pathway in C57BL/6 mice induced by 3% DSS solution [14].
Andrographis paniculata (Burm.f.) Nees
Andrographis paniculata (A. paniculata) a significant medicinal plant in the Ayurveda system, has been employed for countless centuries. Andrographolide (AND), the plant's main active ingredient, is anti-inflammatory, antibacterial, antitumor, anti-obesity, and anti-diabetic. In a randomized, double-blind, placebo-controlled trial, 224 adults with mild to moderate UC found A. paniculata (HMPL-004) was effective. Participants got 1,200 or 1,800 mg of A. paniculata or a placebo for eight weeks. By the eighth week, 45% of 1,200 mg patients and 60% of 1,800 mg patients had clinical responses. 1,800 mg outperformed the placebo and clinical remission rates did not vary. Despite the same side effects, mild to moderate UC patients responded better to 1,800 mg of A. paniculata per day than a placebo [15]. The administration of andrographolide sulfonate aids in alleviating TNBS-Induced Colitis in mice. TNBS-related bodyweight loss, myeloperoxidase activity, colon shortening, and colonic inflammation improve significantly with treatment. It suppresses CD4+ T cell infiltration and the development of Th1 and Th17 subsets, lowering inflammation and suppressing p38 mitogen-activated protein kinase and the p65 component of NF-kB [16]. In a double-blind, placebo-controlled study, A. paniculata plant extract (HMPL-004) prevented T cell-dependent colitis. HMPL-004-treated mice had less intestinal inflammation and weight loss than Methyl Cellulose-treated mice. HMPL-004 suppressed CD4+ T cell replication and diversification and reduced inflammatory cytokines such as TNF-α, IL-1, IFN-Υ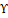 , and IL-22 [17]. The impact of AND, the primary active compound present in A. paniculata extract, on T cell responses in individuals diagnosed with UC, which is an inflammatory bowel condition that is associated with T cell responses. AND dose-dependently reduced interferon, IL-23, and IL-17A while increasing IL-4. Flow cytometry reported a decrease in Th1 and Th17 cells and increased Th2. Andrographolide decreased T-bet and RAR-related orphan receptor t while enhancing GATA-3. In healthy donors' peripheral blood mononuclear cells, we identified effects analogous to those seen in IL-23-mediated T-cell responses. AND may cure IL23-mediated illnesses [18]. In mice, the anti-inflammatory compound CX-10 (3, 14, 19-triacetyl andrographolide) showed promise as a complementary and alternative medication for UC. It reduced UC symptoms like weight loss and colon damage, lowered colonic inflammatory markers, and inhibited NF-kB and MAPK pathways [19]. In another study, AND-activated AMPK in macrophages prevented LPS-induced inflammation and acute colitis in mice [20]. Paniculin 13TM reduced the inflammatory response by lowering dysbiosis, body weight loss, MPO, TNF-α
, and IL-22 [17]. The impact of AND, the primary active compound present in A. paniculata extract, on T cell responses in individuals diagnosed with UC, which is an inflammatory bowel condition that is associated with T cell responses. AND dose-dependently reduced interferon, IL-23, and IL-17A while increasing IL-4. Flow cytometry reported a decrease in Th1 and Th17 cells and increased Th2. Andrographolide decreased T-bet and RAR-related orphan receptor t while enhancing GATA-3. In healthy donors' peripheral blood mononuclear cells, we identified effects analogous to those seen in IL-23-mediated T-cell responses. AND may cure IL23-mediated illnesses [18]. In mice, the anti-inflammatory compound CX-10 (3, 14, 19-triacetyl andrographolide) showed promise as a complementary and alternative medication for UC. It reduced UC symptoms like weight loss and colon damage, lowered colonic inflammatory markers, and inhibited NF-kB and MAPK pathways [19]. In another study, AND-activated AMPK in macrophages prevented LPS-induced inflammation and acute colitis in mice [20]. Paniculin 13TM reduced the inflammatory response by lowering dysbiosis, body weight loss, MPO, TNF-α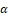 , and iNOS gene expression, without side effects in histology report. Lentilactobacillus kefiri SGL 13 and Paniculin 13TM, isolated from A. paniculata, were also tested for their effects on DSS-treated mice [21].
, and iNOS gene expression, without side effects in histology report. Lentilactobacillus kefiri SGL 13 and Paniculin 13TM, isolated from A. paniculata, were also tested for their effects on DSS-treated mice [21].
Azadirachta indica A. Juss
In the ancient Indian system of medicine, the drug neem, which is also known as Azadirachta indica (A. indica), is considered to be of major importance because it is employed in the treatment of a broad range of conditions. In rats with experimental colitis produced by A. indica, the ingestion of ethanolic extracts of A. indica leaves at a dosage of 500 mg/kg led to a lessening in colon inflammation and damage, improvements in diarrhea and fecal frequency, as well as an increase in body weight. Even at large doses, the A. indica extract did not cause any adverse effects, though exhibiting antibacterial activity in the intestines, increased antioxidant levels, and reduction in free radicals and myeloperoxidase activities with evidence of the extract's safety [22]. The 50% ethanol extract of A. indica (500 mg/kg) dried leaves effectively repairs colitis with TNBS-induced in rats by reducing inflammation, diarrhea, and colonic damage, as well as raising antioxidants with decreasing free radicals and myeloperoxidase activities in colonic tissue. This was accomplished by enhancing antioxidants in colonic tissue and increasing the amount of A. indica [23].
Boswellia serrata Roxb.
For centuries, the gum resin derived from the Boswellia serrata (B. serrata) plant has been utilized to address inflammatory conditions such as arthritis, hyperglycemia, asthma, and cancer. A total of 108 outpatients with CD who were currently experiencing clinical remission participated in the randomized, double-blind, placebo-controlled study at 22 different locations in Germany. Patients were given either Boswelan (3×2 capsules/day; 400 mg each) or a placebo for fifty-two weeks. The research showed that B. serrata had some efficacy and was comparable to large doses of mesalamine in terms of its effectiveness. On the other hand, B. serrata did not show any significant improvement over placebo for patients who had inactive CD [24]. Because of its high antioxidant content, B. serrata extract is an excellent treatment for acute UC in rat models where it was caused by AA. When compared to the colitis group, the treated groups had greater anal sphincter pressure, reduced edema, and preserved mucosal crypts when the B. serrata extract was given orally before and after generating colitis. In addition, there was a significant drop in lipid peroxidation, a decrement in the activity of the SOD enzyme, and an increase in the activity of glutathione peroxidase and glutathione enzyme in the groups that were given treatment [25]. In a rat model of acute UC induced by AA, administration of a B. serrata extract exhibited encouraging anti-inflammatory and antioxidant properties, as evidenced by a substantial reduction in lipid peroxidation, nitric oxide, and iNOS levels when the rats with UC were pre-treated and subsequently treated with the extract. Additionally, there was an improvement in tissue injury and anal sphincter pressure. Overall, the B. serrata extract actively reduced inflammation and oxidative stress in affected rats, which provided protection and enhanced tissue health [26]. The effects of B. serrata oleo-gum extract (BSE) and its derivative acetyl-11-keto-β-boswellic acid (AKBA) were examined in terms of cell viability and barrier function. This evaluation was conducted by exposing colonic epithelial cell monolayers to H2O2 or INF-Υ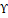 and TNF-α
and TNF-α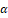 . This was done after the cells had been exposed to either H2O2 or INF- Υ
. This was done after the cells had been exposed to either H2O2 or INF- Υ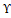 and TNF- α
and TNF- α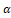 . Both BSE and AKBA were completely risk-free and didn't produce any negative side effects. In addition, there was a considerable reduction in ROS in the pre-treated samples, which led to the protection of functional and morphological changes that were generated by inflammatory stimuli. Based on these findings, it appears that the antioxidant activity of BSE has a favorable correlation with the preservation of the coherence and function of the intestinal epithelium [27].
. Both BSE and AKBA were completely risk-free and didn't produce any negative side effects. In addition, there was a considerable reduction in ROS in the pre-treated samples, which led to the protection of functional and morphological changes that were generated by inflammatory stimuli. Based on these findings, it appears that the antioxidant activity of BSE has a favorable correlation with the preservation of the coherence and function of the intestinal epithelium [27].
Myrrh
Commiphora molmol (C. molmol) is an extract from the secretory tissues on the bark of the Burseraceae family. A year-long randomized, double-blind, double-dummy research evaluated the remission-maintaining efficacy of myrrh, chamomile, and coffee charcoal to mesalazine in UC patients. The herbal treatment was well-tolerated and safe, and the two groups had similar mean CAI and recurrence rates. 96 inactive UC patients participated [28]. Short-chain fatty acids regulate gut immunity with a decrease in intestinal dysbiosis. In another study, the same author examined 38 patient fecal samples through gas chromatography. Within the context of a flare, mesalamine exhibited a reduction in total short-chain fatty acids and butyrate, whereas the herbal preparation (consisting of Myrrh, Chamomile, and Coffee charcoal) did not show such an effect [29]. Myrrh extract protects and cures ethanol-induced stomach ulcers in rats better than C. molmol oil. Myrrh supplementation before or after ethanol-induced gastric ulceration promotes gastric mucosal growth and repair. This is because myrrh boosts the level of the transcription factor Nrf2 as well as the gastric antioxidant potential. This allows myrrh to effectively reduce the negative oxidative, inflammatory, and apoptotic processes associated with ethanol-induced gastric ulcers [30]. Histopathology findings showed that C. molmol oil and extract prevented the development of gastric lesions in rats, as well as maintained the integrity of the gastric epithelium and accelerated the healing process [31].
Curcuma longa L.
Curcuma longa (C. longa) which is more commonly called turmeric or Indian saffron, is known as a versatile herbal medicine and a key ingredient in many traditional medical systems. Neutrophils, which are crucial in innate immune responses, can exacerbate inflammation in IBD. Curcumin demonstrates protective effects in IBD mouse models and reduces inflammation in UC by decreasing neutrophil recruitment to inflammatory sites, influencing chemokine gradient formation, and directly affecting neutrophil polarization, chemotaxis, and chemokinesis [32]. Following treatment with prednisone and curcumin, a woman in her 60s who had a history of left-sided UC and enteropathic arthropathy for 17 years observed an improvement in her chronic colitis and remained in clinical remission for the duration of her medication. Curcumin may be more beneficial for UC than for CD, even though its mechanism of action in treating colitis has not been determined but shares similarities with sulfasalazine [33]. Administration of the Curcuma extract resulted in direct and indirect myorelaxant effects on the ileum and colon of mice. Mice treated with C. longa extract exhibited reduced spontaneous contractions in both the ileum and colon, as well as diminished responsiveness to carbachol compared to untreated mice. Additionally, curcuma enhanced motility in mice with colitis; however, chronic treatment diminished the animals' contractions [34]. Demethoxycurcumin (DMC), is one of the active curcuminoids derived from C. longa, substantially lowered NO secretion by 35–41% and down-regulated iNOS at the mRNA and protein levels in the inflamed cell model, perhaps through powerful suppression of the iNOS pathway [35]. IBD is a condition that lasts for a long time and cannot be cured. It is caused by the improper functioning of numerous genes. The extract and fractions derived from C. longa demonstrate the potential to decrease aberrant cell transport and boost the activity of gene promoters associated with anti-inflammatory cytokines. This was especially true in the case of the IL-10 variation, which was highly connected with the curcumin concentration [36]. The administration of C. longa in rats with AA-induced colitis demonstrated protective effects, including increased body weight, reduced ulcer count, and decreased levels of myeloperoxidase and IL-23 in the colon. Additionally, C. longa was shown to boost serum glutathione levels, which may assist in lowering the oxidative stress associated with colitis [37].
Cyperus rotundus L.
Cyperus rotundus L. (C. rotundus), or nutgrass belongs to the Cyperaceae family and has attracted substantial attention as a medicinal plant due to its wide range of phytoconstituents and use in treating a variety of disorders. Oral administration of an ayurvedic preparation including five different herbs, including C. rotundus, was given to fifty patients with UC for four weeks as part of an observational clinical research that was not randomized. The patients saw a decrease in the frequency of their bowel motions, as well as a lessening of the stomach pain they were experiencing. In addition to this, they reported an improvement in their well-being as well as a decrease in the number of aminosalicylates they consumed [38]. Additionally, another study indicated that the chloroform extract of C. rotundus can drastically diminish the expression of IL-6 and IFN-Υ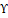 in IBD. The primary constituents of C. rotundus, such as terpenoids and steroids, contain anti-oxidant and anti-inflammatory capabilities, which can lead to the downregulation of proinflammatory cytokines [39]. Anti-inflammatory capabilities are possessed by a sesquiterpene called isocyperol, which may be found in the rhizomes of C. rotundus. By modulating the signaling of NF-kB, STAT3, and heme oxygenase-1, isocyperol can suppress the formation of NO, PGE2, and proinflammatory cytokines in RAW 264.7 cells. Isocyperol exhibits the ability to decrease serum levels of NO, PGE2, and IL-6, leading to an elevated survival rate among mice experiencing septic shock [40].
in IBD. The primary constituents of C. rotundus, such as terpenoids and steroids, contain anti-oxidant and anti-inflammatory capabilities, which can lead to the downregulation of proinflammatory cytokines [39]. Anti-inflammatory capabilities are possessed by a sesquiterpene called isocyperol, which may be found in the rhizomes of C. rotundus. By modulating the signaling of NF-kB, STAT3, and heme oxygenase-1, isocyperol can suppress the formation of NO, PGE2, and proinflammatory cytokines in RAW 264.7 cells. Isocyperol exhibits the ability to decrease serum levels of NO, PGE2, and IL-6, leading to an elevated survival rate among mice experiencing septic shock [40].
Glycyrrhiza glabra L.
Licorice, or Glycyrrhiza glabra L. (G. glabra) a small evergreen herb holds significant importance in treating various conditions within diverse traditional medicinal practices. Glycyrrhizinic acid, a bioactive component of G. glabra, was demonstrated to significantly suppress IBD and reduce oxidative stress in experimental animal models. This was accomplished by inhibiting the production of prostaglandins, a type of protein that is responsible for IBD [41]. When glycyrrhizic acid is given to rats, it offers a significant degree of shielding from TNBS-triggered colitis. This is accomplished by the compound's immunoregulatory effect, which reduces the production of pro-inflammatory IL-6 simultaneously by raising the production of anti-inflammatory IL-10. This action illustrates the compound's ability to control inflammation. When compared to the oral administration of glycyrrhizic acid, the activities of the rectal administration of this compound are superior in terms of their immunoregulatory effects. Additionally, it lowers inflammatory indicators and lessens the intensity of symptoms associated with colitis [42]. By reducing the levels of pro-inflammatory cytokines and chemokines in the inflamed mucosa, rectal administration of the glycyrrhizin formulation at a dose of 2 mg was shown to ameliorate the effects of DSS-induced colitis in rats. IL-1, IL-6, TNF-α, and Cinc-2 were some of the cytokines and chemokines that were engaged. In addition to that, the activity of MPO was suppressed [43]. In the DSS- triggered UC mice model, the effective administration of GR (100 mg/kg/day) led to beneficial outcomes including reduced weight loss, increased colon length, decreased cytokine levels, minimized microscopic colon damage, as well as lowered levels of IL-6 and IL-1. Furthermore, GR administration regulated the phosphorylation of NF-kB and IkB and modulated COX-2 and PGE2 expression in the colon [44]. Glycyrrhizin exerts an influence on the inflammatory response that shows promise when TNBSA-induced colitis in mice models. As a consequence of this, there is a decrease in inflammation, as well as a reduction in the synthesis of inflammatory mediators such as HMGB1, IFN-Υ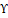 , IL-6, TNF-α
, IL-6, TNF-α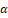 , and IL-17. Glycyrrhizin can regulate dendritic cell and macrophage responses. It limits their ability to drive the maturation of Th17 cells and reduces the replication of Th17 cells [45]. GutGard®, a flavonoid-rich extract of G. glabra, significantly enhanced the barrier function in rats with TNBS-triggered colitis. This was accomplished by preserving the integrity of the intestinal epithelial barrier, modulating tight junction proteins, reducing levels of TNF-α and myeloperoxidase, and increasing levels of secretory IgA. This was achieved by preserving the coherence of the intestinal epithelial barrier, modulating tight junction proteins, and reducing levels of TNF-α
, and IL-17. Glycyrrhizin can regulate dendritic cell and macrophage responses. It limits their ability to drive the maturation of Th17 cells and reduces the replication of Th17 cells [45]. GutGard®, a flavonoid-rich extract of G. glabra, significantly enhanced the barrier function in rats with TNBS-triggered colitis. This was accomplished by preserving the integrity of the intestinal epithelial barrier, modulating tight junction proteins, reducing levels of TNF-α and myeloperoxidase, and increasing levels of secretory IgA. This was achieved by preserving the coherence of the intestinal epithelial barrier, modulating tight junction proteins, and reducing levels of TNF-α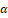 and myeloperoxidase [46].
and myeloperoxidase [46].
Green tea
Epigallocatechin-3-gallate (EGCG), a powerful antioxidant, is abundantly present in green tea. It is being studied for its possible health advantages, which include qualities that fight inflammation and cancer. In a mouse model of colitis provoked by oral administration of DSS, treatment with EGCG demonstrated a positive impact by reducing ROS and peroxides, decreasing neutrophil count, enhancing the antioxidant capacity in colonic tissue, decreasing the secretion of IL-8, and inhibiting inflammatory signaling pathways [47]. This effect was further amplified when piperine was combined with EGCG in the treatment. In mouse models of UC and CD, the administration of green tea polyphenols (GrTP), EGCG, and sulfasalazine dramatically improved colonic damage and histological scores in the treated animals. The treatment led to a substantial decline in inflammatory markers TNF-a, IL-6, and serum amyloid A, which had been raised in animals with colitis. This highlights the demand for safer solutions like EGCG or GTE as sulfasalazine, a conventional IBD medication, entails the risk of severe side effects. EGCG or GTE are two examples of safer options [48]. Polyphenon E, a green tea extract high in EGCG (divided doses of either 400 mg or 800 mg of total EGCG per day), showed promising effects on individuals diagnosed with mild to moderate UC in a pilot study. When compared to the placebo, Polyphenon E resulted in a higher response rate (66.7% vs. 0%) and active treatment remission rate (53.3% vs. 0%). Because it resulted in a therapeutic benefit with relatively minor side effects, Polyphenon E could be a novel therapy option for patients with mild to moderately active UC unresponsive to current medications [49]. A rat model of colitis caused by TNBS, catechins, and specifically EGCG, demonstrated anti-inflammatory and antioxidant properties, stabilized mast cells, and improved UC symptoms. The generation of inflammation-inducing substances such as TNF-α, IL-6, and ROS was decreased by EGCG, which also stabilized the mast cells and dramatically improved the ameliorated the tissue damage in the colon mucosa. This improvement was accomplished by suppressing the activation of NF-kB, which was made possible by EGCG [50]. When compared to pure EGCG, the EGCG-NPs that were synthesized through the process of self-assembling EGCG with ovalbumin (OVA) had better stability and bioavailability. These nanoparticles (NPs) can be taken up by macrophages, which enables them to keep the equilibrium of inflammatory components. As a result, these NPs demonstrated excellent therapeutic efficiency in a DSS-triggered UC animal model [51]. The collection which included curcumin, polyphenols from green tea, and selenium, was found to be an effective therapy for UC in both animals and humans. In this combo, selenium demonstrated significantly higher levels of activity than green tea. The supplement was well accepted and shown to be helpful, with 70% of patients showing signs of improvement, 45% reaching complete remission, and a considerable reduction in the clinical activity index. Despite this, some patients chose to withdraw from the trial or had flare-ups during their participation [52]. It was shown in another study that giving green tea enhanced the wealth of Flavonifractor Plautii (F. Plautii) in the gut microbiota. This study looked at the immune regulatory effects of F. Plautii on a mouse model of colitis. They also gave F. Plautii to mice by mouth for ten days, which resulted in a reduced degree of inflammation and blocked IL-17 signaling, which in turn led to reduced levels of intestinal inflammation. It was determined that lipoteichoic acid derived from F. Plautii was the key constituent that was responsible for IL-17 inhibition [53]. Green tea with a high concentration of the antioxidant EGCG can be effective in regulating the severity of colitis as a result of aging. To achieve this goal, the progression markers of the disease, such as the DAI score, the response of Th1 cytokines, the antioxidant capacity, the tight junction genes, and the cell cycle inhibitors, must be decreased. Consumption of EGCG has been demonstrated to improve the ability to neutralize free radicals, minimize oxidative and inflammatory impairment, and stimulate expression of cell cycle inhibitory genes, all of which result in decreased severity of colitis caused by DSS. Additionally, EGCG consumption has been shown to stimulate the expression of cell cycle inhibitory genes. The onset and severity of colitis may be postponed as a result of these effects, which will ultimately result in a lower risk of carcinogenesis [54].
Moringa oleifera Lam.
The Moringa oleifera Lam. (M. oleifera) trees are native to the Sub-Himalayan regions of India. Traditional wisdom suggests that a combination of M. oleifera tree roots and Citrus sinensis Linn. fruit peel, in equal proportions, has beneficial effects on bowel health. The ethanol extracts from the roots of M. oleifera and the fruit rind of Citrus sinensis produced effects that were comparable to those of the conventional medication prednisolone. In mice with AA-induced UC, the combination was successful in lowering levels of MPO and MDA in the serum and colon tissue of the mice. It would appear from this that the combination possesses an antioxidant effect, which means that it can neutralize reactive oxygen species and thereby reduce inflammation in the colon. Furthermore, treatment with M. oleifera root extracts led to an improvement in histological features as well as a diminish in neutrophil infiltration, which was demonstrated by a suppression of colon MPO levels [55]. It has been demonstrated that regular consumption of the biologically active fraction of M. oleifera can minimize the amount of oxidative stress experienced in the colon. To achieve this goal, the expression of COX-2, as well as the release of IL-8 and MCP-1, and the production of ROS by IL-1-activated Caco-2 cells, must be decreased. The antioxidant and anti-inflammatory properties of M. oleifera may be due to the high levels of β-carotene, vitamin C, and vitamin A that are found in M. oleifera [56]. Moringa seed extract contains a phytocompound namely moringa isothiocyanate-1 (MIC-1), which not only has anti-inflammatory qualities but also exhibits antioxidant capabilities. It was established that MIC-1 reduced levels of colonic pro-inflammatory biomarkers, fecal lipocalin-2 levels, pro-inflammatory cytokines, and iNOS expression in models of acute and chronic UC produced by DSS. In addition to this, it increased the synthesis of phase II detoxifying enzymes and tight junction proteins in the colon, both of which are controlled by the Nrf2 gene. It was established that MIC-1 was effective since it was able to lower the severity of acute and chronic UC pathological recurrence. There is some evidence to show that the anti-inflammatory and antioxidant actions of MIC-1 are the consequence of anti-inflammatory and antioxidant signaling that is mediated by Nrf2 [57]. When given to mice suffering from DSS-induced colitis, the M. oleifera polyphenol extract (MOPE) displays a therapeutic effect. Patients whose condition is treated with MOPE show improvements in several clinical parameters, including a reduction in weight loss, disease activity, colon shortening, mucosal damage, immune cell infiltration, and inflammatory cytokines; also, the patients experience fewer inflammatory cytokines. Moreover, it decreases the generation of proteins that are linked with the NF-kB signaling system, which is an indication that it could be beneficial in the management of inflammatory disorders [58]. The administration of M. oleifera leave extracts to mice that were suffering from colitis associated with colon cancer helped ease the symptoms. This is performed by lowering the level of inflammation and increasing the enzymatic activity of the enzymes GST and NQO1 found in the liver and colon [59]. M. oleifera polysaccharide, more commonly referred to as MOP, is an effective treatment for UC because it lowers inflammatory levels and promotes tight junction secretion. This combination of beneficial properties makes MOP a popular choice. In addition, MOP treatments have the potential to act as a functional diet in the prevention of UC by facilitating the growth of probiotics and controlling the metabolism of polyunsaturated fatty acids. This would be done in the same way as described before. According to the findings of studies involving metabolomics and transcriptomics, MOP appears to be responsible for the control of the metabolism of polyunsaturated fatty acids and may include the PPAR-ϒ, TLRs, and TNF-α signaling pathways. This appears to be the case because MOP appears to contain all three of these pathways [60]. The administration of M. oleifera polysaccharide (MOP) influences the immunological repertoire, as well as the serum and immune organ indices, as well as the microbiota of the colon, in C57BL/6 mice. This effect is because M. oleifera polysaccharide (MOP) changes the components of the microbiota in the colon. The outcomes revealed that MOP exhibits good impact on the immune system and intestinal health in C57BL/6 mice, as it improved thymus and spleen indices, decreased IL-6 and TNF-α levels, regulated the proportion of colonic microflora, and regulated the proportion of microflora in the colon. Additionally, it improved thymus and spleen indices and decreased IL-6 and TNF-α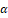 levels [61].
levels [61].
Nigella sativa Linn.
Nigella sativa (N. sativa) is a plant species commonly known as black cumin and belonging to the Ranunculaceae family. Forty-six patients with mild to moderate UC were randomly assigned to receive either 2 grams of N. sativa powder per day or a placebo for six weeks [62]. While the treatment did affect several inflammatory markers, it did not have a meaningful impact on oxidative stress, clinical symptoms, or quality of life. Alginate microcapsules effectively transport N. sativa extract (NSE, Thymoquinone, TQ) to the colon for IBD treatment. Direct encapsulation outperforms the diffusion loading method in terms of loading and efficiency. Microcapsules (40-60 µm) hinder NSE release in simulated gastric and intestinal fluids, yet release 80% of NSE in simulated colonic fluid after eighteen hours. Oral administration of alginate-encapsulated NSE showcases antioxidant activity and successfully delivers TQ to the colon [63].
Pepper spp.
In mice with AA-induced UC, a combination therapy consisting of Amaranthus roxburghianus hydroalcoholic extract and piperine was shown to effectively reduce ulceration, bleeding, necrosis, and leucocyte infiltration. This therapy also lowers myeloperoxidase and malondialdehyde levels while raising glutathione levels in blood and tissue, with the protective effects primarily attributed to the presence of complex phytoconstituents [64]. Piperine effectively alleviates AA-induced UC symptoms in mice, halts colon shortening and spleen enlargement, and decreases inflammation by blocking pro-inflammatory mediators such as NO, TNF-α, and FFA-induced TLR4-mediated inflammation, indicating its potential as a treatment for inflammatory bowel disease in colorectal regions [65]. Piperine, found in black and long pepper, effectively reduces inflammation and may potentially treat IBD. By inducing the Pregnane X Receptor (PXR) and promoting CYP-3A4 gene expression, piperine can potentially prevent and inhibit colonic inflammation through the activation of CYP450 expression via PXR receptor activation [66]. The methanolic seed extract of Piper nigrum effectively reduces ulcer index and epithelial layer infiltration in rats at a 30 mg/kg dose, showcasing its promising potential to treat AA-induced UC by inhibiting inflammatory mediators [67].
Plantago ovata Forsk.
Psyllium husk, which can also be referred to as Isabgol or Plantago ovata Forsk (P. ovata), is an essential component in Ayurvedic medicine. It is well-known for its ability to effectively treat chronic constipation and diarrhea. Albino rats with AA-induced UC showed improvement after receiving an oral administration of aqueous rosemary extract (10 mg/kg B.W.) and psyllium seed husk powder (5 mg/kg B.W.). These treatments were found to be effective and significant improvements in blood parameters, including increased levels of GSH, CAT, and IL-10, as well as decreased levels of NO and IL-1 in serum [68]. Maqliasa, a Persian traditional medication containing P. ovata, was administered to thirteen outpatients with active UC as an adjunct to their standard treatment regimens for twenty-eight days as part of a clinical experiment that was neither randomized nor controlled before or after it took place. Patients were evaluated using the Lichtiger colitis activity index at three different time points. Compared to baseline values, significant improvements in the index were seen after two and four weeks in patients [69]. Flaxseed and psyllium seed oils show promise as alternative therapies in rats with acute alcohol-induced UC by reducing the oxidative stress and inflammatory marker levels in colon tissues, improving hematological parameters, and improving the structural and histological changes in the colon [70].
Polygonum multiflorum Thunb.
Polygonum multiflorum Thunb (P. multiflorum), a traditional Chinese medicinal herb, contains 2,3,5,4'-Tetrahydroxystilbene-2-O-D-glucoside (THSG), which is extracted from the rhizome of the plant. In mice with AA-induced UC, administration of the polyphenolic compound THSG results in a significant reduction in inflammation. This is accomplished by increasing the quantities of PPAR- mRNA as well as the protein, blocking the NF-kB pathway, and reducing the overexpression of inflammatory mediators such as TNF-α, IL-6, and COX-2. Because the effects of THSG on PPAR- mRNA expression are even more significant than those of mesalazine, this compound offers a great deal of potential as a promising option for the treatment of IBD [71]. In a DSS-induced model of UC utilizing BALb/c mice, treatments with THSG significantly increased body weight decreased DAI scores, and restored both epithelial barrier structure and colon histology. These findings were obtained by observing a paradigm in which the administration of DSS resulted in the formation of UC. In addition, treatments with THSG were shown to lessen the severity of histopathologic scores and to raise levels of the tight junction proteins occludin and ZO-1. THSG showed promise as a possible therapeutic drug by increasing levels of the anti-inflammatory cytokine IL-10 while simultaneously reducing levels of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. THSG treatments brought about an improvement in the homeostasis of the gut microbiota in a dose-dependent manner. This led to a rise in the levels of the family Lachnospiraceae_NK4A136 and a decrease in the levels of the genera Helicobacter, Bacteroides, and Parabacteroides [72].
Punica granatum L.
Pomegranates, scientifically known as Punica granatum (P. granatum), are grown mostly in the Mediterranean. It has been used to treat infections, inflammation, diarrhea, and ulcers. PG juice (400 mg/kg body weight, orally) and purified punicalagin (4 mg/kg, orally) for eighteen days reduced the severity of DNBS-induced colitis in rats and reduced the expression of inflammatory cytokines like TNF-α, IL-1, IL-18, and nuclear factor kappa-light-chain-enhancer of activated B cells. These findings suggest that PG juice may cure IBD better than punicalagin alone [73]. P. granatum peel extract (PGE, 100 mg/kg, b.w.) reduced colonic mucosal damage, adhesions, and weight gain in rats with AA-induced colitis. PGE's active ingredients ellagic acid, ellagitannins, and punicic acid flavonoids make it risk-free and antioxidant. In colitis patients, PGE reduced digestive tract microorganisms that damaged tissue and slowed recovery [74]. The efficacy of P. granatum on TNBS-induced colitis in male Wister rats, with the therapeutic group receiving 5 and 8 ml/kg and the preventative group receiving 5 ml/kg. Myeloperoxidase, glutathione, alkaline phosphate, fibrinogen, and C-reactive protein levels changed significantly in the prophylactic group treated with P. granatum at 5 and 8 ml/kg, but only the therapeutic group at 8 ml/kg. P. granatum can treat and prevent inflammation, with greater doses affecting metabolic markers [75]. Pomegranate peel ethanol extract was tested on mice colons in a DSS-induced chronic inflammation. The extract treatment reduced inflammation and inhibited COX-2 and iNOS production in the colons. Additionally, the extract was equally effective as aspirin and pure ellagic acid [76]. A. marmelos and P. granatum treated DSS-induced colitis in mice. The combination of A. marmelos and P. granatum improved all variables except histology, although the high dose of either plant had no effect. The plant medications with low sulfasalazine doses were equally efficacious as those with the standard dose [77]. After 18 days of treatment, UC patients had improved symptoms in a normal colonoscopy and digital rectal examination, proving that the traditional Persian remedy "Sahj," which contains P. granatum, was effective for UC and mucosal problems [78]. In a clinical trial of Ramak, a traditional Persian medicinal compound herbal preparation for intestinal ulcers and recurrent diarrhea, 40 UC patients were randomly to take Ramak or placebo capsules for ten weeks. The study found that Ramak improved general health, symptom aggravation, and defecation urgency while keeping a stable SCCAI total score [79]. Pomegranate peel extract at 480 mg/kg/day may have reduced colorectal cancer by increasing caspase-3 expression and apoptosis [80]. Supplementing the treatment of 30 UC patients with Sahj capsules (2 capsules every 8 hours, 3 times daily, an hour before a meal) for 4 weeks resulted in improvements in SCCAI score, bowel frequency, urgency of defecation, and stool blood, suggesting its potential as a complementary treatment for UC. However, more research is needed to confirm its safety and efficacy [81].
Terminalia spp.
Terminalia chebula Retz. (T. chebula) is a member of the Combretaceae family and is praised for its astringent and aperient characteristics. The plant's unripe and ripened fruit are both utilized to promote gastrointestinal health due to their rich tannin and flavonoid content. In addition to displaying antioxidant and anti-inflammatory capabilities, the dried fruit pulp extract of T. chebula showed therapeutic effects against AA-induced colitis in rats. These effects were seen in addition to the extract's ability to prevent colitis. T. chebula showed healing capabilities in terms of colonic damage score and weight, effectively moderating inflammation and mucosal damage in the colon. This was accomplished through the use of T. chebula. The levels of SOD, CAT, and GPx are increased by the use of T. chebula extracts, which is accompanied by a reduction in the levels of LPO and NO. Tannins, phenolic compounds, and triterpenoids are a few examples of the abundant phytochemicals that may be found in T. chebula extracts. These phytochemicals are responsible for the extracts' positive biological effects [82]. Rats with TNBS-induced colitis recover completely after receiving oral treatment of the T. chebula fruit pulp extract. It was discovered that a dose of T. chebula fruit pulp extract equalling 600 mg/kg was the optimal level for achieving the desired effects of reversing stool output, healing colonic injury, and promoting weight gain. In addition to this, demonstrated a substantial antibacterial action, increased antioxidants, and a reduction in both acute inflammatory markers and free radicals that are responsible for the delayed healing seen in TNBS colitis patients colitis [83]. KM1608, a herbal formulation consisting of Zingiber officinale, T. chebula, and Aucklandia lappa, was able to effectively reduce the severity of colitis symptoms in TNBS-induced colitis. Additionally, it was able to regulate inflammatory responses in the colon by decreasing levels of myeloperoxidase, TNF-α, and IL-6 in the lysate of colon tissues treated with KM1608 [84]. Both the Terminalia arjuna hydroalcoholic extract (TAHA) and the traditional Ayurvedic formulation Arjunarishta have features that make them suitable treatments for IBD. Treatment reduces oxidative cell damage and cytotoxicity as well as antioxidant and antibacterial properties. Both Arjunarishta and TAHA demonstrated antibacterial action against IBD-associated clinical isolates, while TAHA's antioxidant capacity was significantly higher than that of Arjunarishta. In addition to this, they have cytocompatibility with normal rat intestinal epithelial cells (IEC-6) as well as mouse fibroblast cells (L929) [85].
Ashwagandha, commonly recognized as Indian ginseng, and scientifically known as Withania somnifera L. (W. somnifera), is an important medicinal plant and a member of the Solanaceae family. The aqueous extract of W. somnifera root (WSRE) showed strong antioxidant activity and positive effects on histopathological parameters when tested in rats with TNBS-induced IBD. A rectally applied gel containing WSRE (1000 mg/kg. b.w.) demonstrated significant mucorestorative efficacy in TNBS-induced rats, showing results comparable to the commercial anti-inflammatory drug Mesalamine [86].
Zingiber officinale Roscoe.
Ginger, also known as Zingiber officinale (Z. officinale) Rosc., is a plant that belongs to the family Zingiberaceae. Ginger's rhizome serves dual roles as a delightful food spice and a traditional Indian herbal remedy, being extensively utilized in both contexts. This versatility is attributed to the abundance of bioactive components present in ginger, contributing to its wide range of pharmacological properties. Ginger volatile oil had the potential to significantly alleviate the symptoms of experimental colitis produced by AA in rats. Ginger volatile oil, which has effects that are comparable to those of the reference drug prednisolone, effectively alleviates the symptoms of experimental colitis in a dose-dependent manner. It does this by lowering the colon weight/length ratio, as well as the severity, area, and index of ulcers. The dose of 400 mg/kg was found to significantly reduce inflammation severity and extent [87]. In a BALB/c mice model with DSS-induced UC, 6-gingerol (6G) actively reverses symptoms, suppresses inflammatory mediators, restores colonic nitric oxide levels and myeloperoxidase activity, and prevents oxidative damage. By inhibiting iNOS expression and reducing NO concentration in colonic tissue, 6G demonstrates its potential therapeutic value for UC [88]. GDNPs 2, (Edible ginger NPs) provide potential benefits for IBD treatment, such as enhancing intestinal repair, reducing acute colitis, preventing chronic colitis and colitis-associated cancer, and improving inflammatory cytokine balance for faster intestinal mucosal injury healing. As a non-toxic and scalable alternative to synthetic nanoparticles, GDNP 2 effectively targets the colon upon oral administration, offering healing benefits without typical limitations like toxicity and production scale. The anti-inflammatory properties of GDNPs 2 can be attributed to their key bioactive compounds, including shogaols and gingerols [89]. In UC therapy, nanoparticles provide benefits like enhanced drug stability, more accurate targeting, and fewer side effects. Nanoparticles carrying the ginger compound 6-shogaol effectively addressed DSS-induced colitis in mice.
Nanoparticles made of PLGA/PLA-PEG-FA containing 6-shogaol were shown to have excellent biocompatibility and a high rate of cell absorption. These nanoparticles were able to modulate both pro-inflammatory and anti-inflammatory factors, which allowed them to reduce symptoms of colitis in a mouse model when they were orally delivered [90]. Rats with DSS-induced colitis showed significant improvement and accelerated wound healing upon receiving the administration of 6-gingerol, 8-gingerol, and 10-gingerol. These gingerols alleviated the symptoms of colitis, increased the activity of SOD, decreased the levels of malondialdehyde and myeloperoxidase, and decreased the concentrations of TNF-α and IL-1 in the serum. Additionally, they hastened the recovery process of mucosal injuries. The antioxidant and anti-inflammatory capabilities of gingerols may be responsible for the potential benefits of these compounds in the treatment of UC. These compounds may also provide new insights into the prevention of colorectal cancer, which is a major consequence of IBD [91]. When given orally at a dose of 100 mg/kg, 6G was able to effectively prevent rectal bleeding, as well as loss of body weight, reduction in colon mass, and lowered hormone levels in mice that had been subjected to DSS toxicity. In addition to this, 6G was able to lower markers of oxidative stress and pro-inflammatory cytokines, all while increasing sperm qualities, testicular function indicators, and testicular histoarchitecture. The antioxidant and anti-inflammatory activities of 6G were improved, and the protection against UC-induced testicular injury was maintained. 6G possesses chemoprotective qualities and has the potential to be a treatment that is both safe and effective for UC-induced damage to human testicles [92, 93]. Another study found that 6G was able to prevent chronic UC by downregulating NF-kB and decreasing pro-inflammatory cytokines. In lipopolysaccharide-induced RAW264.7 cell models and TNBS-induced colitis rat models, the anti-inflammatory effects of the supercritical fluid extract (AZ-SFE) of traditional Chinese herbs Angelica sinensis and Z. officinale were observed. The treatment with AZ- AZ-supercritical fluid extract prevented the generation of NO, which in turn controlled the immunological response of Th1, alleviated disease activity, reduced oxidative stress, and regulated hepcidin and serum iron levels [94]. The researchers gave either a placebo or a dose of dried ginger powder equivalent to 2,000 mg per day to each of the 46 participants in a 12-week randomized, placebo-controlled trial of patients with mild to moderate UC. After six and twelve weeks of treatment, ginger significantly decreased MDA levels without reducing the total antioxidant capacity of the serum. Ginger supplementation was shown to enhance patients' quality of life throughout the trial [95]. Gingerol nanoparticles with covalent links to chitosan that were between 50 and 78 nm in size were able to efficiently encapsulate 5-amino salicylic acid and allow for its delayed, controlled release. This favorable controlled release of 5-ASA at gastrointestinal pH revealed remarkable promise in treating IBD, as evidenced by an entrapment efficiency that was greater than 50%. The chitosan-bound ginger-derived nanocarriers did not exhibit any cytotoxicity towards fibroblast cells [96]. Jangkanghwan (JKH), a traditional Korean cuisine consisting of 12 components, one of which being dried ginger, exhibits natural anti-inflammatory qualities that aid in reducing inflammation brought on by DSS toxins in mice. In mice, JKH not only prevents weight loss and promotes weight gain during the healing process, but it also slows the rate at which the colon gets shorter. In addition to this, it is responsible for regulating both pro-inflammatory and anti-inflammatory factors in serum and tissues, as well as controlling the murine disease activity index. In addition, JKH alters the composition of the gut microbiome by lowering the proportion of bacteria belonging to the Phylum Bacteroidetes, raising the proportion of bacteria belonging to the Phylum Firmicutes, and increasing the proportion of bacteria belonging to families such as Bifidobacteriaceae, Lactobacillaceae, and Akkermansiaceae [97]. Ginger polysaccharides (GP) have been shown to possess anti-inflammatory and immune-regulating characteristics, which may be beneficial in the treatment of UC. Using a mouse model of UC that was induced by DSS, we found that GP at a dose of 200 mg/kg alleviated symptoms, inhibited pro-inflammatory cytokines, regulated gastrointestinal immune responses, repaired the intestinal barrier, and modulated intestinal microbiota. In addition, treatment with GP brings the ratio of firmicutes to bacteroidetes in the makeup of the gut microbiome into balance, raises the levels of Lactobacillus and Verrucomicrobiota, and decreases the levels of proteobacteria and Bacteroides [98]. Traditional Chinese medicines Z. officinale and Panax ginseng work together to treat UC synergistically by encouraging the growth of good bacteria while simultaneously inhibiting the growth of harmful bacteria. A possible mechanism that could be behind the synergistic effects of this herb pair in the treatment of UC is the modulation of the gut microbiota-metabolite axis [99].
CONCLUSION
Medicinal plants provide a rich source of bioactive compounds for drug discovery, with their diverse chemical structures and traditional use offering valuable insights. However, challenges such as isolating and characterizing natural compounds, complexity, sustainability concerns, and ethical sourcing due to overexploitation make the process lengthy, expensive, and hard to optimize. Today, IBD treatment depends on strictly regulated immunosuppressive or hormone drugs, which can lead to side effects and drug resistance if misused. Although not fully acknowledged by doctors, herbal medicine is becoming increasingly popular among IBD patients due to greater access to products and information, and it can influence the immune system and traditional drug metabolism through various interactions. This review presents a unique approach to exploring the therapeutic potential of bioactive compounds for IBD treatment. Our literature review highlights medicinal plants such as Aegle marmelos, Aloe vera, Andrographis paniculata, Curcuma longa, Glycyrrhiza glabra, Green tea, Moringa oleifera, Punica granatum, and Zingiber officinale, along with their preparations possessing strong anti-colitis properties and acting through different mechanisms against various toxins. The frequent use of herbal remedies by IBD patients calls for evaluating their effectiveness, safety, quality, and marketing, informing stakeholders of benefits and risks, and rigorously studying medicinal plants as potential supplements or alternatives for IBD treatment.
ACKNOWLEDGMENTS: The author is indebted to the Department of Pharmacy, Faculty of Engineering and Technology, Annamalai University, Annamalai Nagar, Chidambaram for providing the necessary support to carry out this literature survey.
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHICS STATEMENT: None