Litsea cubeba (Lour.) Pers. belongs to the family Lauraceae, which occurs mainly in the tropical and sub-tropical regions worldwide. The plant gained importance for its essential oils, sesquiterpenoids, flavonoids, lignans volatile oils, and numerous secondary metabolites. The Essential oil extracted from its bark, stem, and leaves can be used commercially for the preparation of medicines, insecticides, perfumes, flavors, and colognes. The secondary metabolites extracted from L. cubeba show potential pharmacological activities, viz., antipyretic, analgesic, antidiarrheal and anti-tumor, antimicrobial, anti-inflammatory, antioxidant, anti-HIV, hepatoprotective, antidiabetic, and hypothermic activities. In the northeast plants and parts are sold in the local market and used by local people for various ailments and culinary purposes. Overexploitation of the plants took place due to their essential oils and medicinal value; therefore, conservation strategies are needed. Here, we are summarizing the medicinal uses of Litsea sp. and the conservation strategies for the Lauraceae family plants using various tissue culture approaches.
INTRODUCTION
Lisea cubeba (Lour.) Pers. famous as may change or mountain pepper (China), macaw (Taiwan) is a fast-growing aromatic, evergreen tree with small white dioecious flowers and pepper-like fruits. It is native to China and other Southeast Asian countries. L. cubeba belongs to the family Lauraceae, which comprises 45 genera and about 2850 species and occurs mainly in tropical and sub-tropical regions around the world [1]. In Arunachal Pradesh, it is commonly known as tayer and has been used by different ethnic groups as an astringent, antiseptic, stimulant, anti-inflammatory, hypertensive, insecticide, worm infection, and bone fracture. The fruits are sold in the market in Arunachal Pradesh, India, to be eaten raw or as pickles [2]. In Assam, it is known as mejankari, and its leaves are used for rearing muga silkworms [3].
In this study, we are summarizing the medicinal uses of Litsea sp. and the conservation strategies for the Lauraceae family plants using various tissue culture approaches.
RESULTS AND DISCUSSION
Botanical description
Leaves alternate, lanceolate, elliptic, or elliptic oblong. Umbellule axillary is solitary in very short corymbs or clusters of 2-7, 4-6 flowered; bracts 4-6, orbicular, glabrous to hairy. Perianth lobes 6, perfect; male flowers: stamen 9-12, in 2 rows, filaments glabrous or hairy; stamina glands stalked or sessile; female flowers staminodes 9-12 in a row, pistil glabrous, stigma dilated, notched at the center, ovary ellipsoid, glabrous. Berries globose to ovate, 4-6 mm in diameter, perianth tube persistent, small, not enlarged, 1-4mm in diameter (Figure 1). Flowering occurs from November to March, and fruiting from February to July [4].
|
|
|
|
a) |
b) |
|
|
|
|
c) |
d) |
|
Figure 1. Litsea cubeba; a) Tree in Sagalee, Arunachal Pradesh, b) Flowering branch, c) Fruits, and d) Fruits sold in market |
|
Phytochemistry of L. cubeba
The chemical compositions present in the plant body, starting with the leaves, stem, and fruits, are better understood thanks to macroscopic and microscopic investigations on L. cubeba. The L. cubeba contains a variety of pharmacologically potent substances. The phytochemical screening of secondary metabolites in different solvent extracts has revealed the presence of phenols, flavonoids, alkaloids, cardiac glycosides, tannins, saponins, and anthocyanins [5]. The major components found in the essential oils extracted from stems, leaves, and flowers were monoterpenes such as Citral (neral), b-phellandrene, geranial, (8z)- Heptadecene and b-terpinene [6-9]. These compounds are attributed to the various pharmacological activities and sweet- lemon fragrance of the flowers [10]. A natural alkaloid, litebamine, obtained from L. cubeba has been studied by many researchers as it possesses acetylcholinesterase activity, anti-thrombotic activity, and prevents cardiovascular diseases [11-14].
Pharmacology of L. cubeba
All parts of the plant yield essential oil, but fruit essential oil is important and has been extensively used to prepare geranial nitriles, ionone, Citral, and vitamins E, K, and A [15, 16]. Raw fruits are used as carminative by the traditional healers of Sikkim [17]. In aquaculture industries, the use of L. cubeba essential oil can reduce the adverse effects of antibiotics, which are extensively used against pathogenic bacteria [18]. Chiou et al. (1998) demonstrated anti-acetylcholinesterase activity using Litebamine N-homologues [14]. Among them, N-metho salt of N-Propylnorthebaine showed the highest activity with IC50 2.70 µM. L. cubeba fruit essential oil possesses neuropharmacological activity and has shown its effect on the central system in mice [8]. Ho et al. [19] reported the cytotoxic activity of fruit essential oil against human lung, liver, and oral cancers. The vapor of L. cubeba fruit oil has a deleterious effect on Akt phosphorylation, which in turn induces apoptotic death and prevents cell proliferation of NSCLC [20]. Previous pharmacological investigations have revealed that this plant has the following properties:
Hypoglycaemic activity
L. cubeba fruits have been used by natives of Sikkim and Darjeeling Himalayas for the treatment of diabetes [5, 21]. The plant is known as siltimut, and one raw fruit is to be taken two times daily for 4-6 weeks to treat diabetes. According to Chakraborty et al. [5], the methanolic extracts of L. cubeba fruits exhibited the highest antidiabetic potential with IC50 values of 514.9 µg/mL and 1435.7 µg/mL in α-amylase and α-glucosidase inhibition assay respectively. The presence of active secondary metabolites such as phenols and flavonoids may contribute to the hypoglycaemic activity of the plant, as well as the digestive enzymes (alpha-amylase and alpha-glucosidase), which are responsible for increasing the blood sugar inhibited.
Vaso relaxing effect
Laurotenanine, an alkaloid isolated from L. cubeba, depicted vasorelaxation of the Rat thoracic aorta [22]. 3-50 µM lauratenanine inhibited the contraction of aortic rings induced by high potassium (60 µM) and cumulative concentration of calcium (0.3-3 mM) with an IC50 value of 19.8 ± 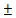 3.6 µM (N = 6) in a 1mM ca2 + medium. Litebamine, another alkaloid from the wood of L. cubeba, exhibited specifically in collagen by inhibiting art aortic SMSs (RASMCs) and A10 thoracic SmCs adhesion to collagen [11]. Litebamine also inhibited platelet-derived growth factor (PDGF)-induced RASMC migration, suggesting its usage in preventing cardiovascular diseases.
3.6 µM (N = 6) in a 1mM ca2 + medium. Litebamine, another alkaloid from the wood of L. cubeba, exhibited specifically in collagen by inhibiting art aortic SMSs (RASMCs) and A10 thoracic SmCs adhesion to collagen [11]. Litebamine also inhibited platelet-derived growth factor (PDGF)-induced RASMC migration, suggesting its usage in preventing cardiovascular diseases.
Antimicrobial (antifungal and antibacterial) activities
Citral present in L. cubeba oil accounted for the disruption of the cell wall and membrane permeability of Magnaporthe grisea [23]. Also, it was found effective against Gibberella zeae, Fusarium oxysporum, Valsa mali, Botrytis cinerea, and Rhizoctonia solani with EC50 values ranging from 39.52 to 193.00 µg/mL. Suhem et al. [24] also reported the antifungal activity of volatile L. cubeba essential oil against Aspergillus flavus. This mold is usually found contaminating brown rice snack bars. The antifungal activity of L. cubeba vapor was improved by laser treatment with Aspergillus flavus inhibition by 80% for at least 25 days. Few endophytic fungi were found to be associated with the leaves and barks of L. cubeba. These endophytes possess antimicrobial activity, and the study conducted by Deka and Jha revealed that these endophytes can be of great use in the pharmaceutical industry as they produce bioactive compounds.
Furthermore, Acremonium falciform, dominant fungi in L. cubeba, showed antagonistic activity against Staphylococcus epidermis (MTCC43). The alkaloidal extract of L. cubeba exerts antimicrobial activity against Staphylococcus aureus [25]. The L. cubeba essential oil has been found effective against Vibrio parahaemolyticus, Listeria monocytogenes, Lactobacillus plantarum, and Hansenula anomala in vitro [26]. Recently, Hu et al. [27] reported the destructive effect of L. cubeba oil on the methicillin-resistant Staphylococcus aureus (MRSA) cell membrane. The L. cubeba oil inhibited the Hexose monophosphate pathway and glucose-6-phosphate dehydrogenase activity. They also reported that citral formed Chimera with MRSA DNA. The essential oil extracted from flesh fruits of L. cubeba was tested for its antibacterial activity against Escherichia coli [28]. 0.125% (v/v) of L. cubeba oil was found to be effective and resulted in the death of most of the E. coli cells within 2 hours. This activity was attributed to Aldehydes present in the oil, which penetrated the cell membrane of E. coli and created holes and gaps, which ultimately led to its death.
Anti-inflammatory activity
The methanolic bark extract and a fraction of L. cubeba inhibited NO and PGE production in LPS-activated RAW 264.7 macrophages, suggesting the anti-inflammatory activity of the plant [29]. The activity of myeloperoxidase catalyzing oxidation of chloride to HOCL and O2 production was significantly reduced by L. cubeba bark extracts and fractions. Geranial and neral, two isomers of citral, obtained from fruit essential oil of L. cubeba, showed antioxidant activity [30]. Compared to geranial, neral demonstrated better anti-inflammatory activity, including significant inhibition of cytokine secretion and inflammatory molecule expression of LPS-stimulated macrophages.
Antioxidant activity
The methanolic extract of L. cubeba exhibited remarkable antioxidant activity and was studied using three different assay systems: DPPH assay, Peroxidase/guaiacol assay, and TBA test [31]. The methanolic extract of L. cubeba showed 90.57 ± 0.07% as the highest scavenging effect on DPPH radicals as compared to other fractions. L. cubeba extract reduced the level of H2O2 in the peroxidize/guaiacol assay, and the MeOH extract, CHCL3 fraction, and BuOH fraction showed 89-90% inhibition of lipid peroxidation in the TBA method. The alkaloid fractions of L. cubeba fruit showed antioxidant activity for DPPH radicals with IC50 values of 219.43 ± 0.43, 242.97 ± 0.93, 92.38 ± 0.17, 40.84 ± 0.04, 103.83 ± 3.29, and 103.75 ± 0.42 µg/mL, respectively [32]. Further, this property was also reported by Chakraborty et al. [5].
Insecticidal activity
The fruit essential oil of L. cubeba was found to be effective against stored-grain insects and can be used as a natural fumigant for pest control. Also, it can be used as an insect repellent and has been reported for species such as Aedes aegypti, Tribolium castaneum, Sitophilus zeamais, Lasioderma serricome, and Liposcelis botrychophila [33-36]. Wu et al. [37] evaluated L. cubeba oil for mosquito repellence against Aedes albopictus. According to them, L. cubeba essential oil can be used as an alternative to chemical pesticides for mosquito prevention due to its low toxicity and environment-friendly nature.
Traditional and ethnobotanical uses
In India, the fruits and seeds are widely consumed either raw or prepared in dishes like pickles and chutneys, enjoyed by various ethnic groups. In Arunachal Pradesh, crushed fruits and leaves mixed with water are taken orally twice daily to treat conditions such as blood dysentery, stomach problems, and fever. The leaves can also be used as a paste on the forehead to relieve headaches. Fresh, ripe, and unripe fruits are used to remedy colds and coughs and improve sleep. These fruits have culinary uses as both food, spice, and condiment. For threadworm infections, the seeds are chewed. Additionally, water-based paste from the bark is applied to treat bone fractures [38]. Within the Chiru tribal community in Manipur, the flowers and fruits are harnessed as remedies to alleviate sore throat discomfort [39]. Similarly, the Indigenous people of the Darjeeling and Sikkim Himalayan region have been using this plant to manage diabetes. In efforts to manage and regulate high blood sugar levels, ethnic groups in this area routinely consume one or two raw fruits, either as chewable or pickled preparations [5, 21].
Industrial applications
L. cubeba finds versatile industrial applications due to its aromatic qualities and chemical composition. Its essential oil is used extensively in aromatherapy and perfumery and as a natural flavoring agent in the food and beverage industry. It's also valued in personal care products and household cleaners and as a potential ingredient in medicinal and therapeutic formulations [8]. Additionally, it serves as a natural insect repellant and contributes to industrial cleaning products [37, 40].
Conservation strategies using tissue culture approaches.
Tissue culture approaches have emerged as a pivotal advancement in plant propagation and cultivation, showcasing distinct advantages over conventional methods in various plant species (Figure 2). In the case of L. cubeba, a plant of immense economic and therapeutic value, the choice of propagation method can significantly influence its growth, yield, and quality. This introduction sheds light on the heightened significance of tissue culture approaches in comparison to conventional propagation methods. The conventional propagation method of L. cubeba is through seeds, but these seeds remain deeply dormant after dispersal, forming long-lived seed reserves [41]. This could be due to the impermeability of ligneous seed coat. Thus, propagation through seeds forms an inefficient method [8] and can be overcome by harnessing the potential of tissue culture; we unlock novel possibilities for the efficient mass production, preservation, and genetic fidelity of this botanical treasure, surpassing the limitations of conventional techniques. Several works regarding micropropagation studies using different explants have already been reported on L. cubeba (Table 1).
|
|
|
Figure 2. Medicinal importance and conservation strategies of L. cubeba |
A system for plantlet regeneration of L. cubeba was established via adventitious bud induction from callus using leaves and stem explants. Stems were reported to be best for callus induction. M.S. medium supplemented with 2 mg/L B.A. and 0.1 mg/L IBA was shown to be most effective for explant induction of L. cubeba. In the meantime, the darkening degree of callus would be mitigated by adding an appropriate concentration of vitamin C to the medium of adventitious bud induction. Moreover, the most favorable medium for root regeneration was ½ MS + 0.2 mg/L IBA + 0.4 mg/L NAA. The structure of calli and the formation of adventitious buds were discovered by histological analysis [42].
Stem sections with buds from the mature plant of L. cubeba were used for explant in the study of organ culture to establish a rapid technique system of micropropagation and to find out the best culture medium of initiation. The rate of initiation was 80%. The rate of propagation could reach about 80%. The rooting rate was about 86.7% [43].
Table 1. Various explants and the medium used for in-vitro micropropagation and somatic embryogenesis for Lauraceace family plants
|
Sl no. |
Plant |
Explants used |
Medium |
Result |
References |
|
1 |
Persea indica |
Seedling axillary bud |
MS + 1mg/l (2.8µM) N6- BA |
Shoot proliferation |
[44] |
|
2 |
Lindera melissifolia |
Shoot cultures |
WPM+ 1µM Zeatin |
Shoot multiplication |
[45] |
|
3 |
Cinnamomum camphora |
Shoot tips |
MS+ 4.44µM BA |
Lateral shoots |
[46] |
|
Shoot tips |
MS+ 1.0 mgL-1 BAP and 2.5 mgL-1 TDZ |
Shoots proliferation |
[47] |
||
|
Leaf sections |
MS + 1.0mgL-1 BAP+ TDZ |
Compact callus |
|||
|
4 |
Cinnamomum tamala |
Immature embryos |
MS+ 12µM BA+ 100mg/L polyvinyl pyrolidon |
Morphogenesis |
[48] |
|
5 |
Laurus nobilis |
Micro cuttings |
MS medium+ BA |
Shoots proliferation |
[49] |
|
6 |
Persea lingue |
Apical sections of micro shoots |
MS medium + 0.1mg/L IBA+ 2.0mg/L BAP |
Direct organogenesis |
[50] |
|
7 |
Litsea glutinosa |
Nodal segments |
MS+ 10.0µMN8--BA |
Callus |
[51] |
|
8 |
Litsea cubeba
|
Stem segments |
MS+ 0.1mg/L 6-BA+0.2mg/L NAA |
Shoots proliferation |
[52] |
|
Leaves and stem |
MS+ 2mg/L BA + 0.1mg/L IBA |
Callus formation |
[42] |
||
|
Stem sections with buds |
|
Direct organogenesis |
[43] |
||
|
|
MS+ 2.0mg/L 2,4-D+ 2.0mg/L 6-BA |
Callus |
[53] |
||
|
Buds |
MS+ 1.5 mg/L BA + 0.2 mg/L IBA+ VC 10mg/L+ 10000 mg/L sucrose |
Secondary buds |
[54] |
||
|
Shoot tip, node, and petiole. |
WPM+ NAA |
Shoots proliferation |
[55] |
||
|
9 |
Ocotea catharinensis |
Globular/ early cotyledonary somatic embryos |
½ WPM+ 20g/L sucrose+ 400mg/L glutamine+ 2g/L phytagel+ 1.5 activated charcoal |
Repetitive embryogenesis |
[56] |
|
10 |
Persea americana |
Somatic embryos |
MMSE medium |
Somatic embryogenesis |
[57] |
|
11 |
Eusidendron zwageri |
Leaf |
½ MS + BAP+ 2,4-D or NAA |
Somatic embryogenesis |
[58] |
According to Yin [53], various concentrations of auxin (2,4-D and NAA) along with proper cytokinin concentration in the M.S. medium can induce calluses from L. cubeba explants. The medium with 2.0 mg/L 2,4-D + 2.0 mg/L 6-BA was optimized for callus growth.
Ling et al. [54] used one-year-old buds on stems of adult L. cubeba as explants to make rapid propagation of the seedlings. The basic medium for L. cubeba tissue culture was recommended as an improved M.S. (MS+ 380 mg/L Ca (NO3)2 + 8.2 mg/L H3BO3), and the proliferating culture was an improved M.S. (MS + 1.5 mg/L B.A. + 0.2 mg/L IBA + V.C. 10 mg/L + 10000 mg/L sucrose). On the condition of 2000- 4000 lux natural scattered light, the period of a subculture of GLLC- 4, GLLC- 3, and GLLC- 1 secondary buds was 28d, and the multiplication coefficients were 5,6,3.1 and 4.8, respectively.
Plant multiplication using the method of successive transfer culture of L. cubeba was developed [59]. The modified M.S. medium supplemented with 1.0 mg/L of 6-BA and 0.2 mg/L of NAA was reported to be an optimal medium for successive transfer cultures. When the concentration of NAA was 0.2 mg/L, the rate of proliferation increased with the increasing 6-BA concentration, while the callus gradually reduced when the 6-BA concentration was 1.0 mg/L.
Chongjian et al. [60] reported that the M.S. medium supplemented with 1.0 mg/L 2,4-D and 2.0 mg/L B.A. induced callus, and the M.S. medium with 0.5 mg/L 2,4-D and 3.0 mg/L 6-BA was best for shooting [60]. A rapid micropropagation system was developed for L. cubeba using various explant sources (shoot tip, node, and petiole) [55]. Woody plant medium (WPM) supplemented with B.A. produced multiple shoots, and the shoot tip and the axillary explants were the only responsive explants. Compared to axillary shoot explants, shoot tip explants produced longer shoots. For rooting, in vitro-produced shoot cuttings were transferred to WPM supplemented with various concentrations of NAA. 0.54 µM NAA gave the best rooting responses.
CONCLUSION
Tissue culture techniques such as micropropagation of rare, endangered, and vulnerable plants can be used to obtain many planting materials without harming the mother plant and its natural habitat, thus conserving biodiversity. Tissue culture can stimulate the production of valuable secondary metabolites, which have medicinal, aromatic, and industrial applications. By optimizing culture conditions, researchers can enhance the yield and quality of these metabolites [61]. L. cubeba is a repository of active secondary metabolites that account for its various pharmacological activities. Almost all the parts of the plant yield essential oil, but the fruit essential oil is of great importance. The L. cubeba fruit essential oil has been used as a skincare agent in southern China. An efficient in vitro protocol is important to acquire a maximal number of plantlets in a minimum period with proper rooting and acclimatization in the field. Somatic embryogenesis for genetic transformation is a reliable method as it has a single-cell origin and reduces the time in acquiring seeds of woody trees, as in the case of L. cubeba, where the seeds remain dormant for a longer period. By harnessing the potential of tissue culture, we unlock novel possibilities for efficient mass production, preservation, genetic improvement, and commercial cultivation.
ACKNOWLEDGMENTS: The authors would like to acknowledge the help and support of the Higher authority of NERIST, Arunachal Pradesh, and the University of Lucknow, Lucknow, Uttar Pradesh for their support and motivation.
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: Authors M.K. and P.K. would like to acknowledge the support of the IERP-GBPH, Government of India (ref. GBPI/IERP/17-18/58) and DBT-Twinning project funded by the Department of Biotechnology, Government of India (ref. BT/PR24741/NER/95/836/2017).
ETHICS STATEMENT: None